W&T Wetenschap & Technologie
Een plek om te discussiëren over wetenschappelijke onderwerpen, wetenschappelijke problemen, technologische projecten en grootse uitvindingen.



SPECIAL WEBCAST: Ever wondered how NASA plans to fly astronauts to Mars? You can find out on June 23rd and 24th, when the Coca-Cola Space Science Center visits Cape Canaveral for a live webcast about NASA's new Space Launch System. Special guests will be announced later today.
www,spaceweather.com
www,spaceweather.com
<a href="http://www.vwkweb.nl/" rel="nofollow" target="_blank">[b]Vereniging voor weerkunde en klimatologie[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>


23-06-2016
Marsjeep vindt verrassend mineraal op rode planeet
© reuters.
De Amerikaanse Marsjeep Curiosity heeft op de Rode Planeet een verrassend mineraal gevonden. Dat meldt het Amerikaanse ruimtevaartbureau NASA.
Bij het onderzoek van een staal gesteente dat de robot een jaar geleden heeft genomen, is tridymiet ontdekt. Dit mineraal is tot nu toe alleen op Aarde gevonden in samenhang met kiezelzuurrijk vulkanisme, bijvoorbeeld aan de St-Helenavulkaan in het noordwesten van de VS. Wetenschappers hadden dergelijk vulkanisme en dus ook niet tridymiet op Mars verwacht.
Volgens NASA-wetenschapper Doug Ming rijst daarmee de vraag of Mars tijdens zijn ontstaan niet heftiger en explosiever vulkanisme heeft gekend dat tot nu toe gedacht.
(HLN)
Marsjeep vindt verrassend mineraal op rode planeet
© reuters.
De Amerikaanse Marsjeep Curiosity heeft op de Rode Planeet een verrassend mineraal gevonden. Dat meldt het Amerikaanse ruimtevaartbureau NASA.
Bij het onderzoek van een staal gesteente dat de robot een jaar geleden heeft genomen, is tridymiet ontdekt. Dit mineraal is tot nu toe alleen op Aarde gevonden in samenhang met kiezelzuurrijk vulkanisme, bijvoorbeeld aan de St-Helenavulkaan in het noordwesten van de VS. Wetenschappers hadden dergelijk vulkanisme en dus ook niet tridymiet op Mars verwacht.
Volgens NASA-wetenschapper Doug Ming rijst daarmee de vraag of Mars tijdens zijn ontstaan niet heftiger en explosiever vulkanisme heeft gekend dat tot nu toe gedacht.
(HLN)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


http://www.jpl.nasa.gov/news/news.php?feature=6542
Normaal kom ik hier niet, maar ik zag toevalligquote:News | June 24, 2016
NASA Weighs Use of Rover to Image Potential Mars Water Sites
Ever since it was announced that there may be evidence of liquid water on present-day Mars, NASA scientists have wondered how best to further investigate these long, seasonally changing dark streaks in the hope of finding evidence of life -- past or present -- on the Red Planet.
"It's not as simple as driving a rover to a potential site and taking a scoop of soil," said Jim Green, NASA's director of planetary science. "Not only are these on steep slopes, we need to ensure that planetary protection concerns are met. In other words, how can we search for evidence of life without contaminating the sites with bugs from Earth?"
Pending approval of a mission extension, NASA's Curiosity Mars rover will continue to climb to progressively higher and younger strata on Mount Sharp, investigating how long the ancient, water-rich environments found so far persisted as Mars dried out. Reaching those destinations would bring the rover closer to locations where dark streaks are present on some slopes. On the way, the route would allow the one-ton rover to capture images of the potential water sites from miles away and see if any are the seasonally changing type.
The features of interest have been observed by NASA's High-Resolution Imaging Science Experiment (HiRISE) camera on the Mars Reconnaissance Orbiter (MRO). They appear as dark lines that appear to ebb and flow over time. Planetary scientists think these gullies or recurring slope lineae (RSLs) may appear seasonally as a form of briny water at or near the surface of the Red Planet under warmer conditions.
There are two RSL candidates that may be within Curiosity's reach, on the side of the 3.1-mile-high (5-kilometer-high) Mount Sharp. The rover's Remote Micro-Imager (part of ChemCam) would be the main instrument for imaging the possible sites. The goal would be to study the regions over time to see if there are any changes and to rule out other causes for the changes, such as dry avalanches.
How close could the rover safely get to an RSL? "That's exactly the question that needs to be addressed early in the process," said Catharine Conley, NASA's planetary protection officer. "Kilometers away -- it's unlikely that it would be an issue. In terms of coming much closer, we need to understand well in advance the potential for Earth organisms to come off the rover, and that will tell us how far away the rover should stay."
Conley notes that while the Martian environment is considered harsh for many organisms, that's not necessarily the case for all of them -- particularly microbes that might be hiding within the nooks and crannies of a robotic explorer.
The darkish streaks are considered "special regions" on Mars, where extra precautions must be taken to prevent contamination because of the suspected presence of liquid water, considered a prerequisite for life.
The Mars Science Laboratory (MSL) spacecraft launched from Cape Canaveral, Florida, on Nov. 26, 2011, arriving on the Red Planet on Aug. 6. 2012. NASA's most ambitious Mars mission to date, its goal was to study the Martian environment and determine if Mars is, or was, suitable for life. A decision on the rover's potential extended mission is expected in the next several months.
en dacht dat dit wel interessant was.twitter:MarsCuriosity twitterde op zaterdag 25-06-2016 om 01:07:46 Possible liquid water sites on Mars? I might get to investigate two on the surface from afar https://t.co/p7HA7Pzl7V https://t.co/P4ZV1pqFGs reageer retweet
Als het niet met een hamer te repareren is, is het een elektrisch probleem.


Wow.. dit is best indrukwekkend als het klopt:
http://www.iflscience.com(...)-atmospheric-oxygen/
http://www.iflscience.com(...)-atmospheric-oxygen/
Klinkt als een sterke indicatie dat er leven op Mars heeft bestaan.quote:Mars Used To Have Earth-Like Levels Of Atmospheric Oxygen
Mars may be a famously red, dusty, thinly insulated planet now, but once upon a time, billions of years ago, it almost certainly had huge seas of liquid water and a warmer climate, not unlike our own world. Now, thanks to another glorious find from the Curiosity rover, researchers can confirm that Mars was even more Earth-like than anyone had ever realized.
Using its ChemCam instrument, which probes the geochemistry of any rock samples it comes across, Curiosity has determined that there are extremely high levels of manganese oxide present in surface-level Martian rocks – at least within the Gale crater in which the little mechanical laboratory is based. In order to get such high quantities of this compound, a planet requires just one thing: plenty of free oxygen floating around the atmosphere.
This means that, when these rocks formed, Mars had an atmosphere somewhat like that of Earth’s. Although it’s difficult to determine precisely how much free oxygen there was from this preliminary data, we now know that it must have been somewhat similar conditions to those that existed between 2 and 2.5 billion years ago, when something called the Great Oxygenation Event (GOE) occurred.
The GOE was most likely caused by the appearance of photosynthesizing life, which slowly converted the carbon dioxide-rich atmosphere on Earth into oxygen. When the surface geology could not chemically react with and subsequently absorb any more free oxygen, the rest was left to build up in the atmosphere, and the world was oxygenated.
Manganese oxide simply cannot form without extremely high quantities of free oxygen in the atmosphere, as observed on our own world at the time of the GOE. This also means that there was abundant liquid water on the surface, which agrees with multiple previous studies.
Niet meer aanwezig in dit forum.


quote:Op zaterdag 25 juni 2016 09:43 schreef Resistor het volgende:
http://www.jpl.nasa.gov/news/news.php?feature=6542
[..]
Normaal kom ik hier niet, maar ik zag toevalligen dacht dat dit wel interessant was.twitter:MarsCuriosity twitterde op zaterdag 25-06-2016 om 01:07:46 Possible liquid water sites on Mars? I might get to investigate two on the surface from afar https://t.co/p7HA7Pzl7V https://t.co/P4ZV1pqFGs reageer retweet
27-06-2016
Marsrover gaat mogelijk naar vloeibaar water zoeken
Foto: ANP
De Amerikaanse ruimtevaartorganisatie NASA overweegt om Marsrover Curiosity naar vloeibaar water te laten zoeken op Mars.
Het onderzoeksvoertuig is op dit moment bezig met de beklimming van Mount Sharp, een 5 kilometer hoge berg in een grote krater.
Naast de berg bevinden zich hellingen waarop donkere strepen zijn waargenomen die duiden op de aanwezigheid van vloeibaar water. Dit gebied ligt binnen het bereik van de Marsrover op een afstand van ongeveeer 5 kilometer.
Dat meldt de nieuwssite van de NASA.
Zout
De ruimtevaartorganisatie hoopt met Curiosity te kunnen onderzoeken of de donkere strepen op ruimtefoto's van Mars echt worden veroorzaakt door water en of er leven aanwezig is in het mogelijke oppervlaktewater.
De breedte van de donkere strepen varieert met de temperatuur op de rode planeet. Bij lage temperaturen zijn ze ongeveer vijf meter breed, bij hoge temperaturen krimpen ze iets. Dat suggereert dat de strepen worden gevormd door een zoute stroom water die reageert op seizoensinvloeden.
De NASA twijfelt echter nog of het verantwoord is om de Marsrover naar een gebied te sturen waar mogelijk water aanwezig is.
Bacteriën
Hoewel het voertuig op aarde grondig is gereinigd, is er een kleine kans dat er nog aardse bacteriën op het wagentje zitten. Wanneer die bacteriën in een omgeving met water terechtkomen, kunnen ze zich in theorie op Mars nestelen.
De wetenschappers willen voorkomen dat de planeet vervuild raakt met aardse levensvormen.
"We kunnen dus niet zomaar een Marsrover naar een plek sturen waar mogelijk water aanwezig is en daar een monster nemen", verklaart hoofdonderzoeker Jim Green. "De vraag is hoe we naar bewijs voor leven kunnen zoeken zonder de plek te vervuilen met bacteriën van de aarde."
Door: NU.nl/Dennis Rijnvis
(nu.nl)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


Very exciting indeedquote:Op dinsdag 28 juni 2016 22:54 schreef Molurus het volgende:
Wow.. dit is best indrukwekkend als het klopt:
http://www.iflscience.com(...)-atmospheric-oxygen/
[..]
Klinkt als een sterke indicatie dat er leven op Mars heeft bestaan.
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


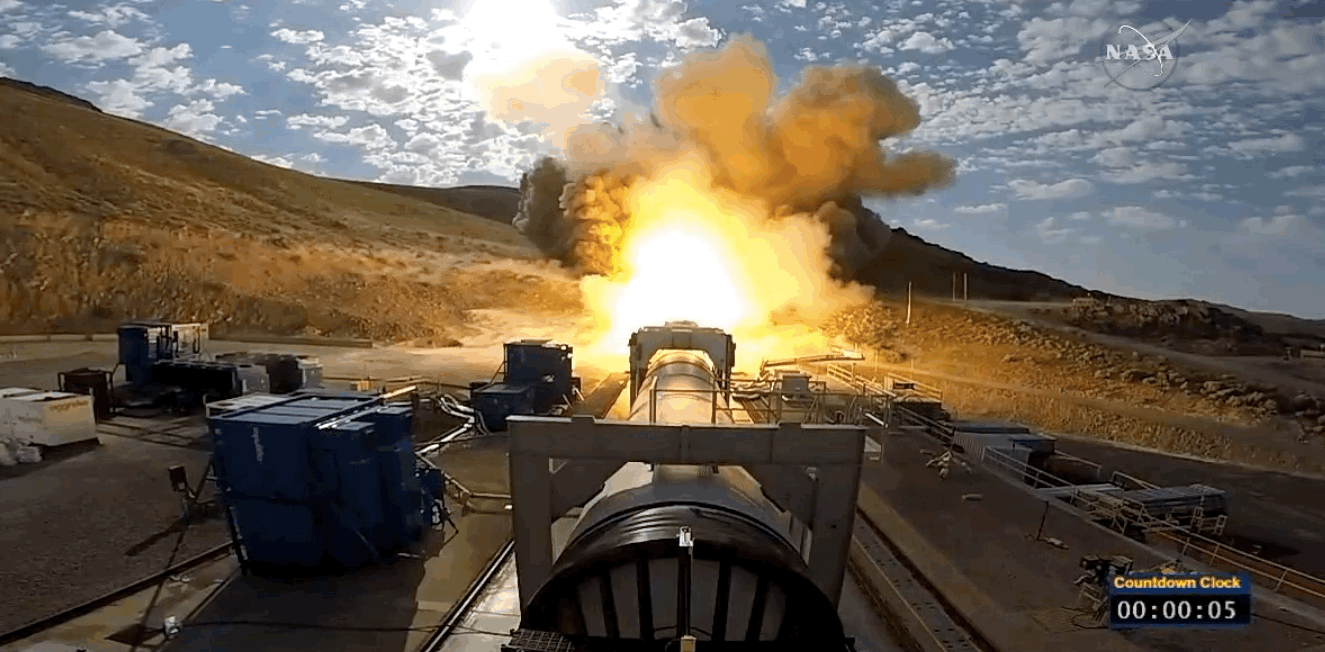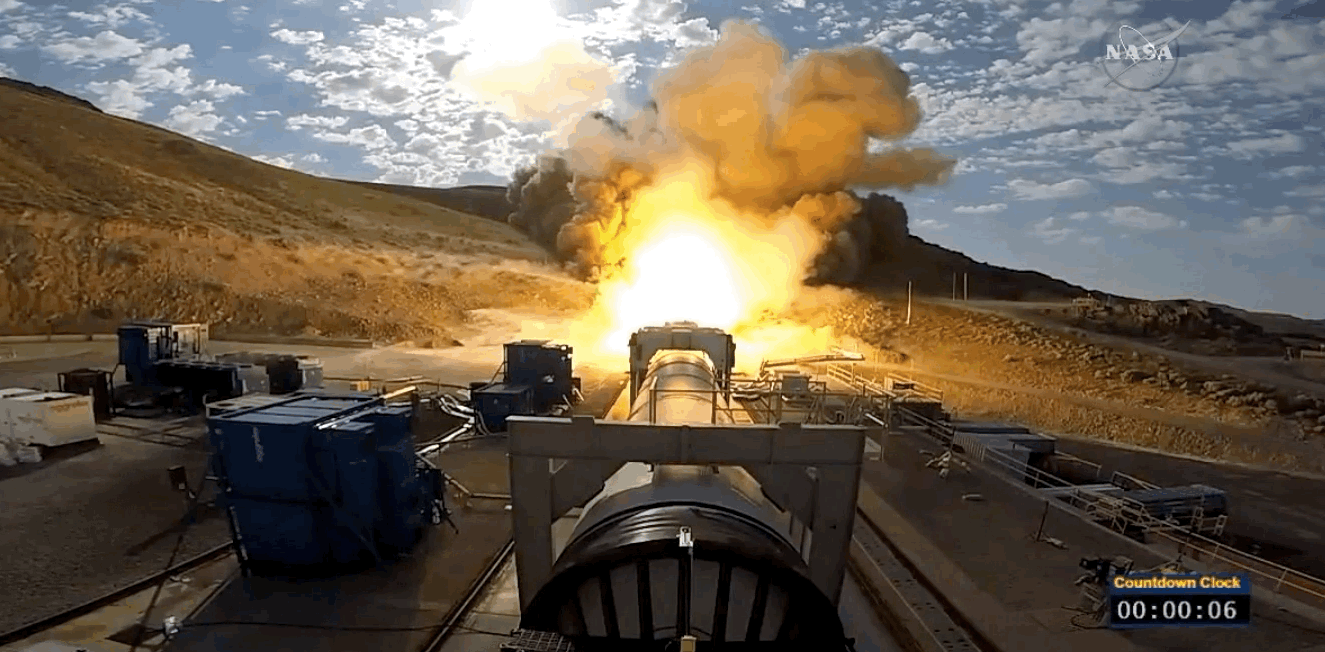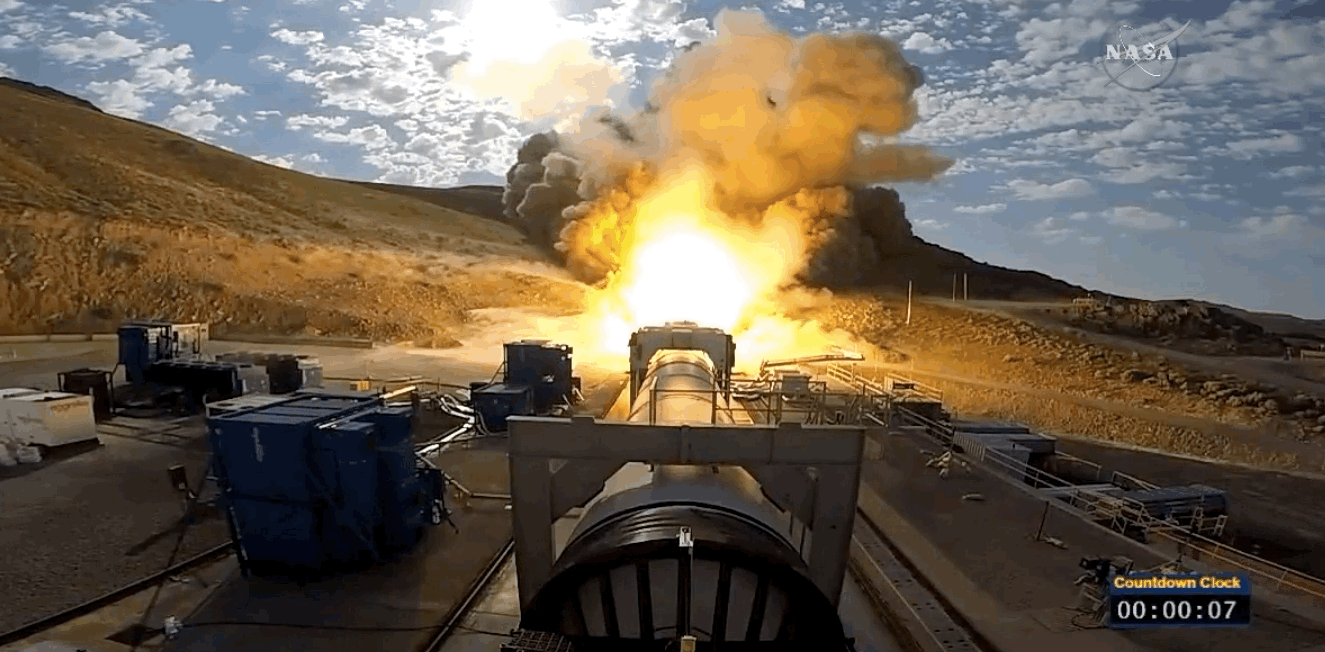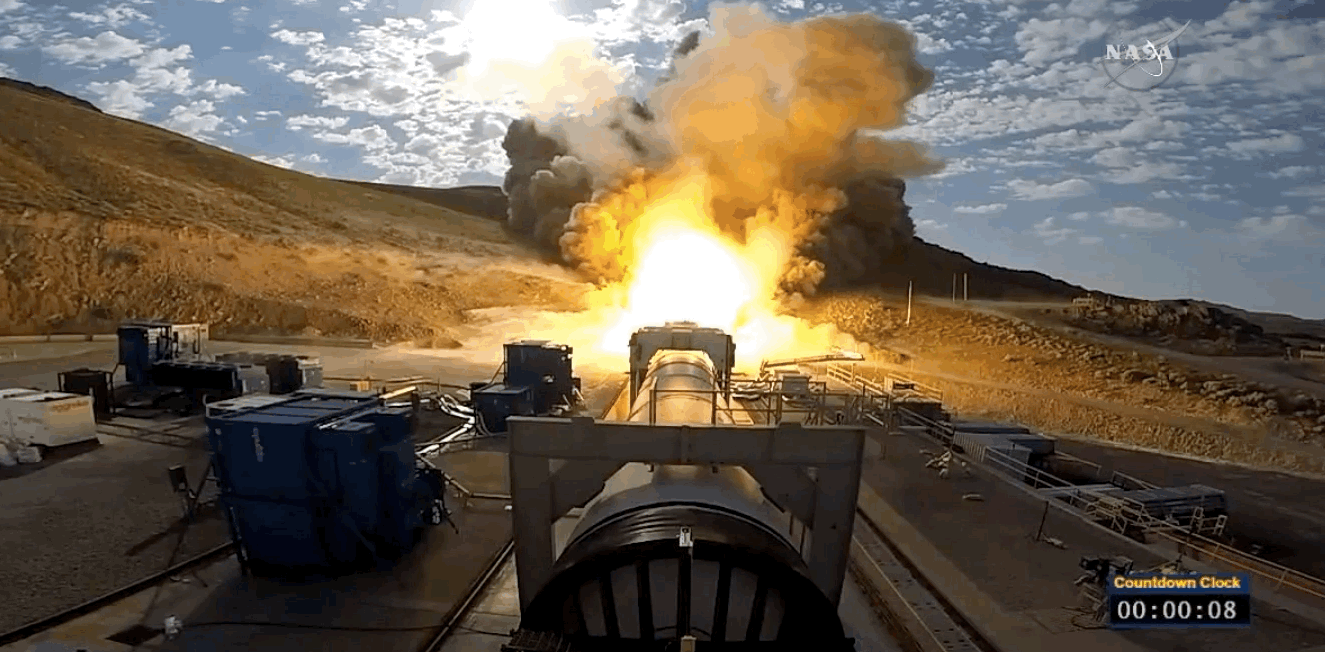
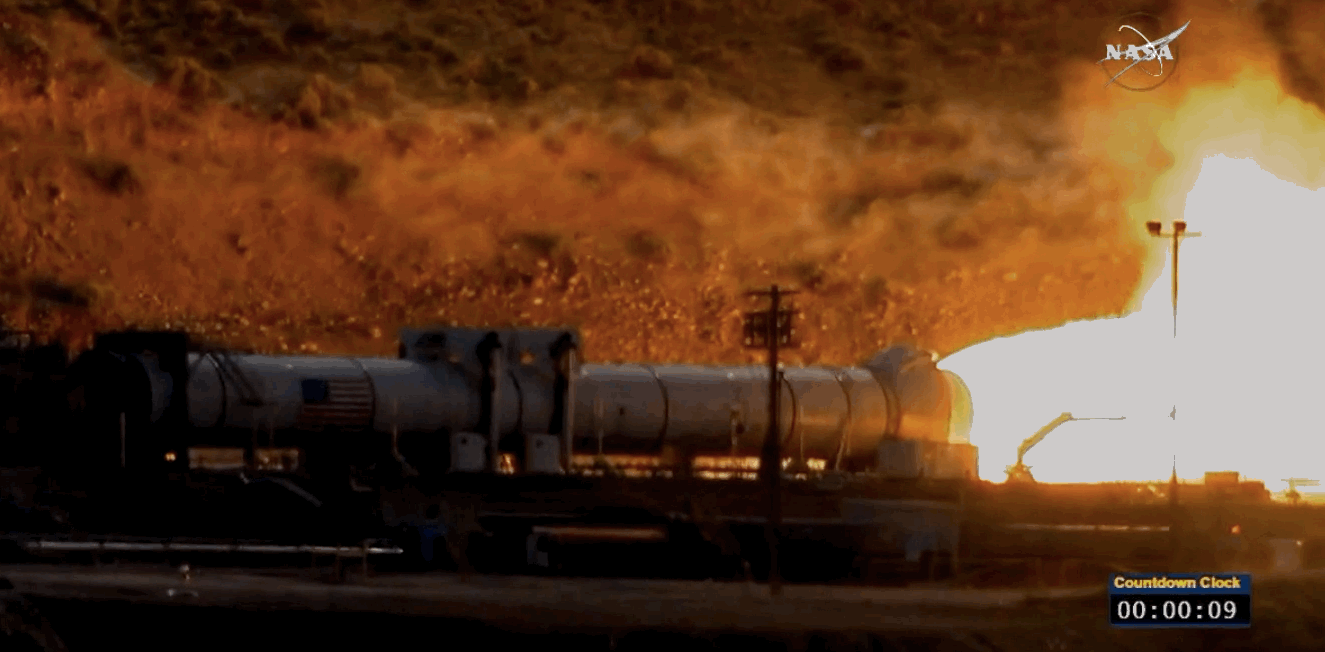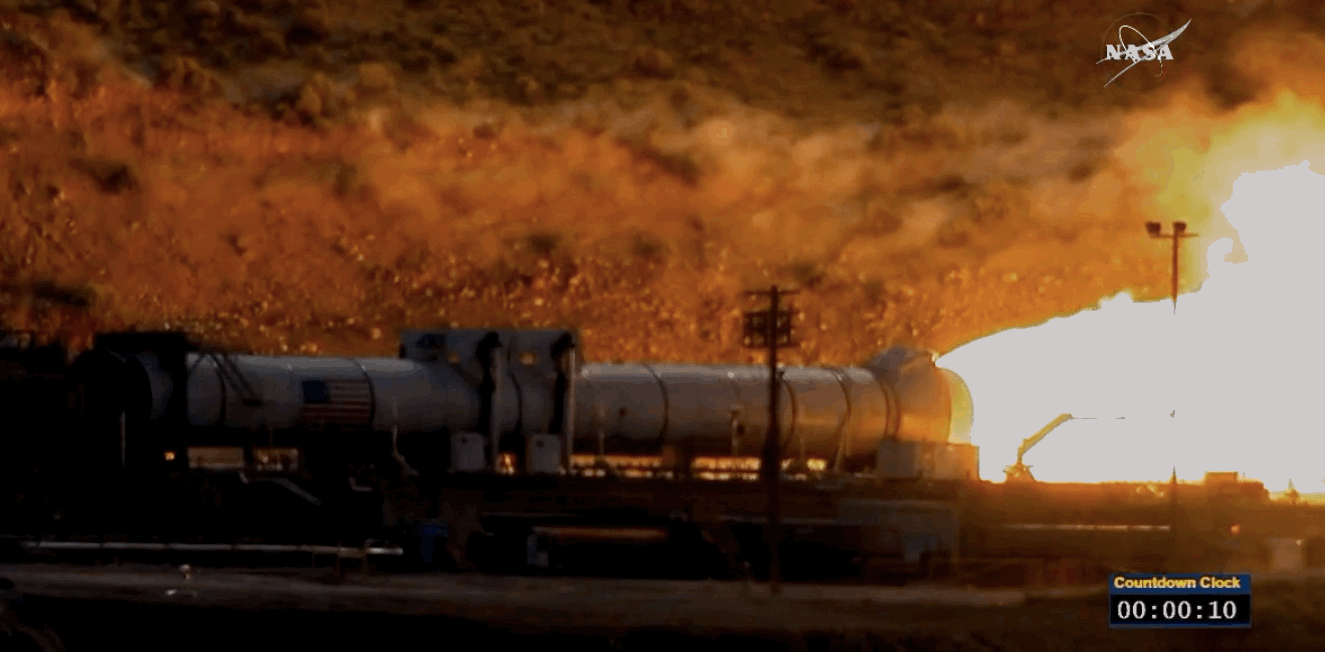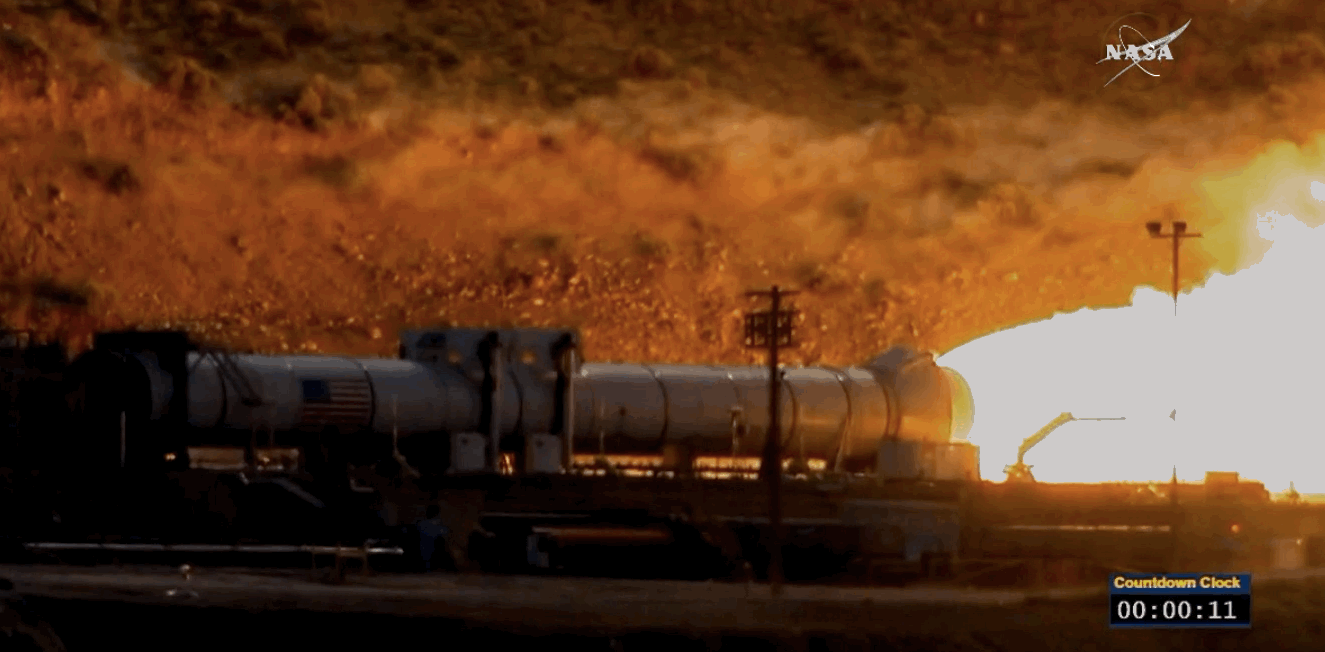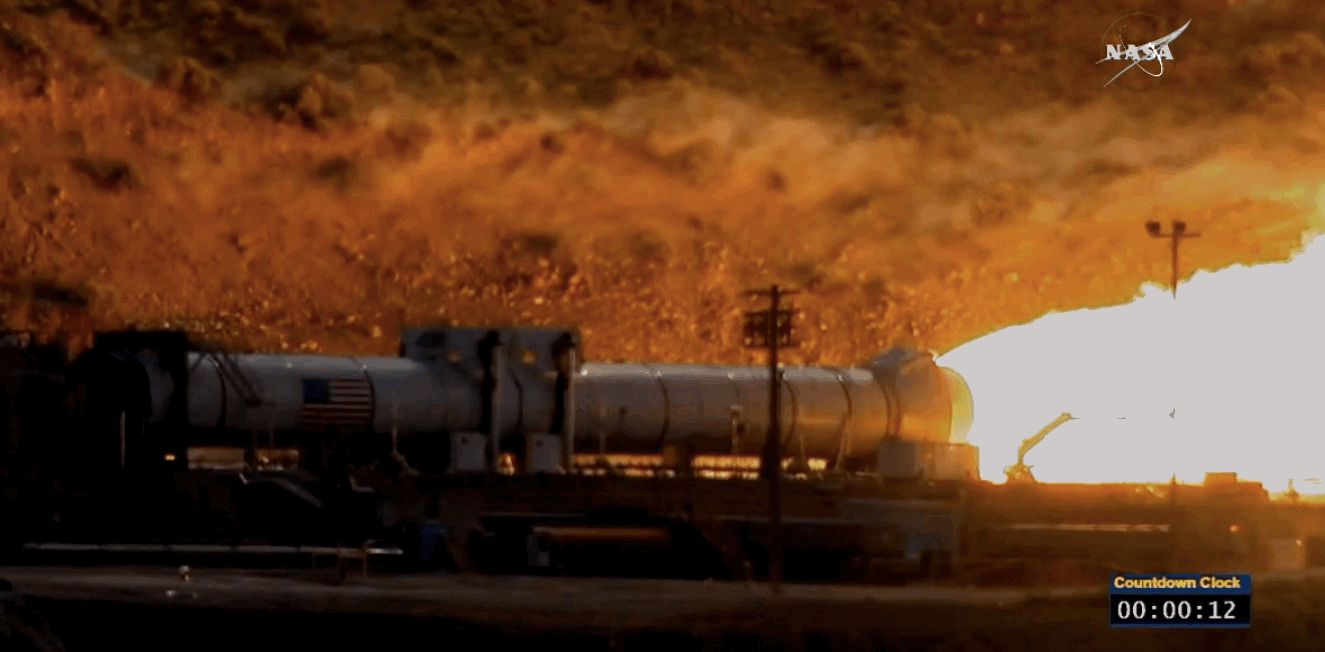
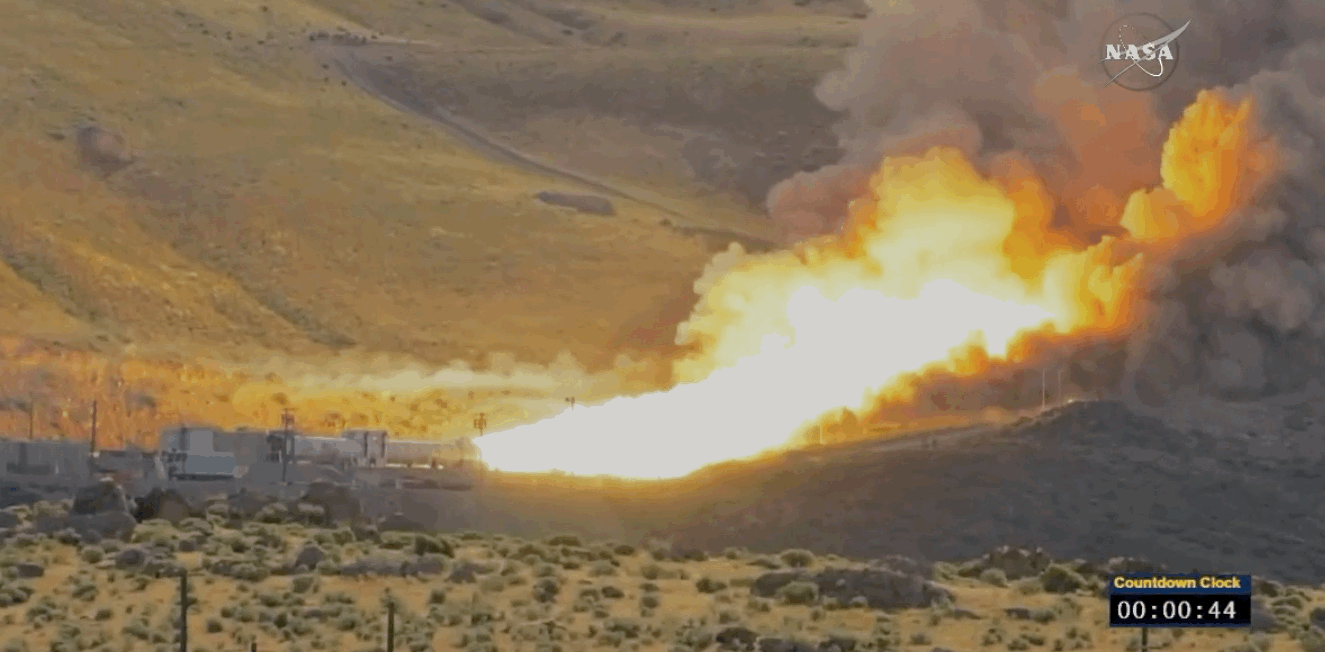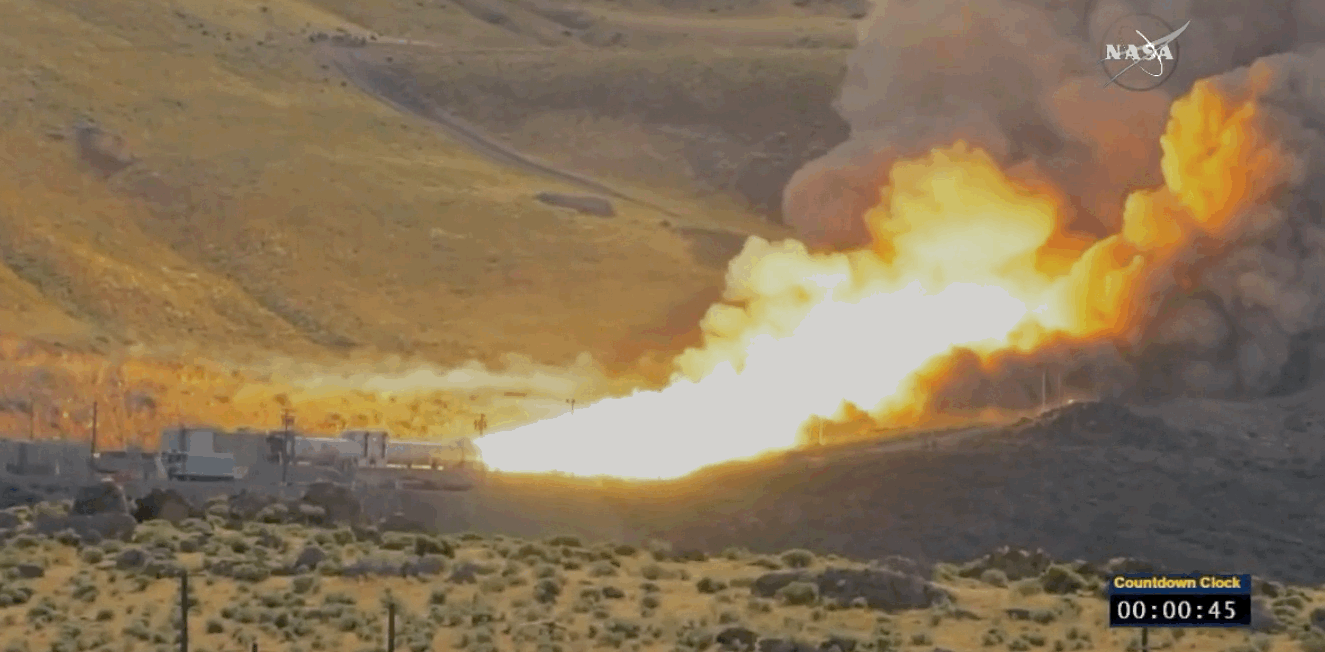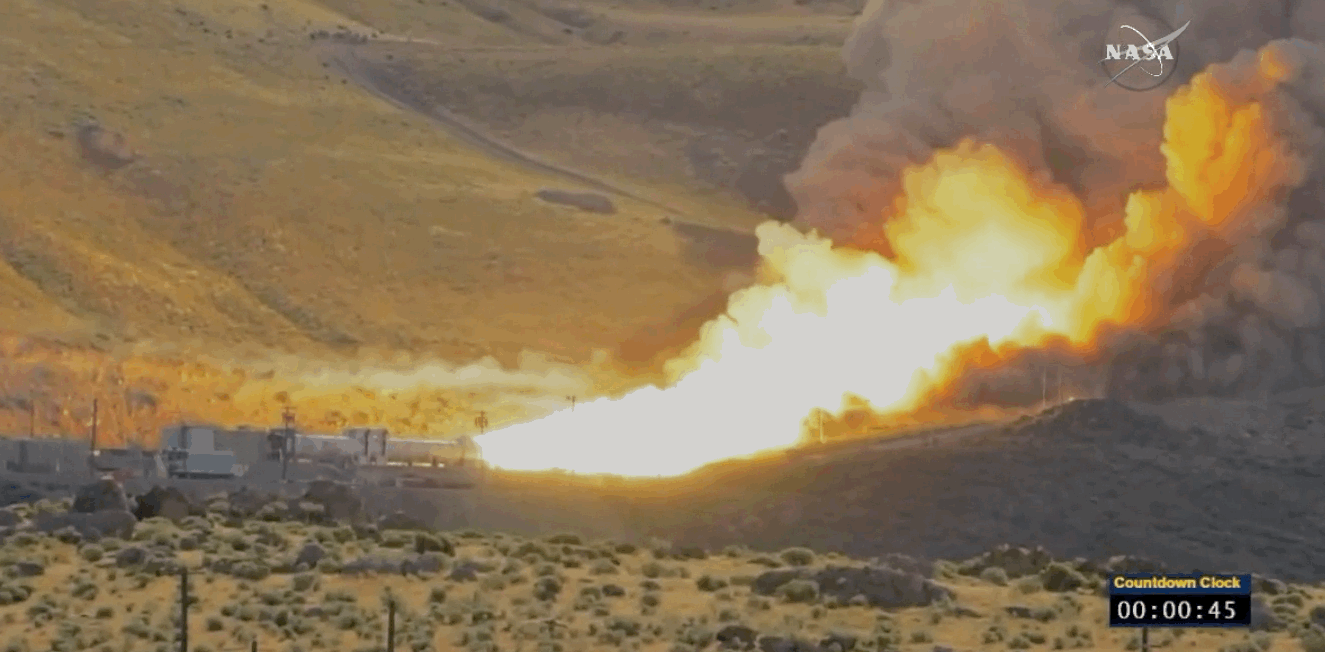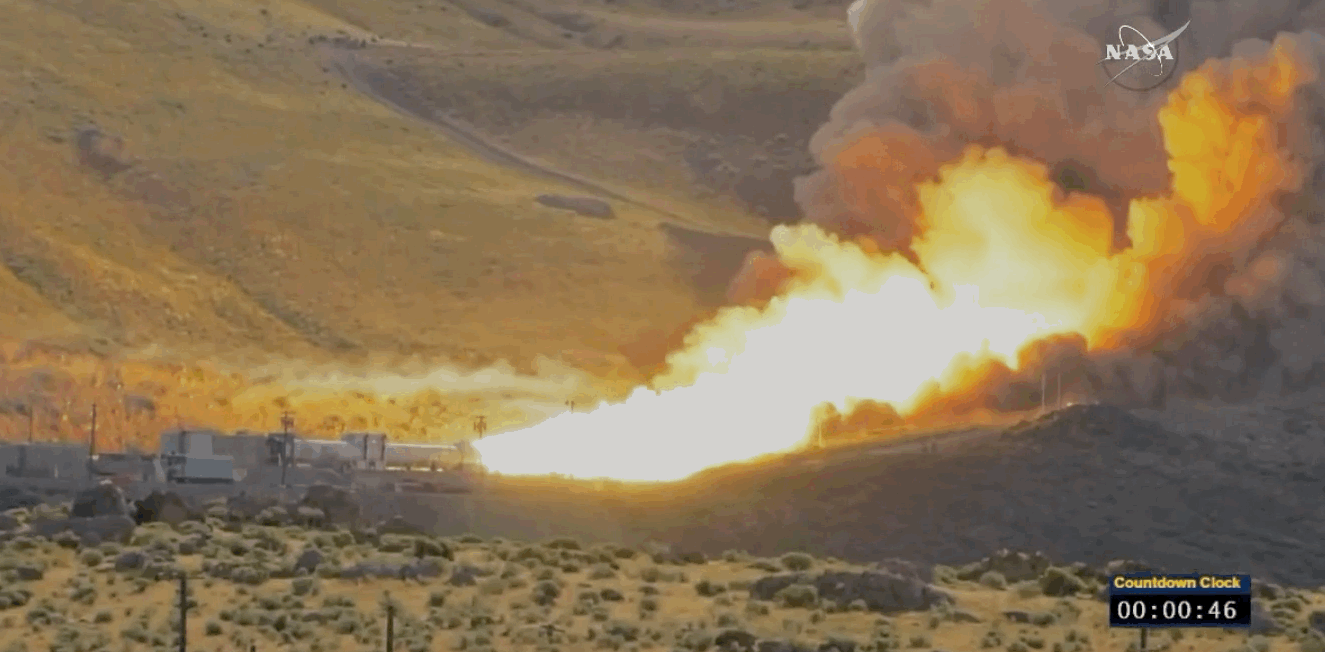
NASA just tested the rocket booster that'll take us to Mars
Last trial before launch.
NASA just passed a huge milestone in its mission to Mars, after conducting the final ground test of one of the boosters that'll power the Space Launch System (SLS) to the Red Planet.
It's the last trial of the system's boosters before the 2018 unmanned launch of the Orion spacecraft, and, as you can see from the footage below, everything looked really good lighting up the middle of the desert.
[ Bericht 5% gewijzigd door Kijkertje op 29-06-2016 23:32:41 ]
“The fundamental cause of the trouble in the modern world today is that the stupid are cocksure while the intelligent are full of doubt.”— Bertrand Russell


quote:Op woensdag 29 juni 2016 23:23 schreef Kijkertje het volgende:
NASA just tested the rocket booster that'll take us to Mars
Last trial before launch.
NASA just passed a huge milestone in its mission to Mars, after conducting the final ground test of one of the boosters that'll power the Space Launch System (SLS) to the Red Planet.
It's the last trial of the system's boosters before the 2018 unmanned launch of the Orion spacecraft, and, as you can see from the footage below, everything looked really good lighting up the middle of the desert.
[ afbeelding ]
[ afbeelding ]
[ afbeelding ]
[ afbeelding ]
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


ChemCam findings hint at oxygen-rich past on Mars
by Staff Writers
Los Alamos NM (SPX) Jul 01, 2016
quote:The discovery of manganese oxides in Martian rocks might tell us that the Red Planet was once more Earth-like than previously believed. A new paper in Geophysical Research Letters reveals that NASA's Curiosity rover observed high levels of manganese oxides in Martian rocks, which could indicate that higher levels of atmospheric oxygen once existed on our neighboring planet.
This hint of more oxygen in Mars' early atmosphere adds to other Curiosity findings - such as evidence of ancient lakes - revealing how Earth-like our neighboring planet once was.
"The only ways on Earth that we know how to make these manganese materials involve atmospheric oxygen or microbes," said Nina Lanza, a planetary scientist at Los Alamos National Laboratory and lead author on the study published in the American Geophysical Union's journal. "Now we're seeing manganese-oxides on Mars and wondering how the heck these could have formed."
Lanza uses the Los Alamos-developed ChemCam instrument that sits atop Curiosity to "zap" rocks on Mars and analyze their chemical make-up. This work stems from Los Alamos National Laboratory's experience building and operating more than 500 spacecraft instruments for national defense, giving the Laboratory the expertise needed to develop discovery-driven instruments like ChemCam. In less than four years since landing on Mars, ChemCam has analyzed roughly 1,500 rock and soil samples.
Microbes seem a far-fetched explanation for the manganese oxides at this point, said Lanza, but the idea that the Martian atmosphere contained more oxygen in the past than it does now seems possible.
"These high-manganese materials can't form without lots of liquid water and strongly oxidizing conditions," said Lanza "Here on Earth, we had lots of water but no widespread deposits of manganese oxides until after the oxygen levels in our atmosphere rose due to photosynthesizing microbes."
In the Earth's geological record, the appearance of high concentrations of manganese is an important marker of a major shift in our atmosphere's composition, from relatively low oxygen abundances to the oxygen-rich atmosphere we see today. The presence of the same types of materials on Mars suggests that something similar happened there. If that's the case, how was that oxygen-rich environment formed?
"One potential way that oxygen could have gotten into the Martian atmosphere is from the breakdown of water when Mars was losing its magnetic field," said Lanza.
"It's thought that at this time in Mars' history, water was much more abundant." Yet without a protective magnetic field to shield the surface from ionizing radiation, that radiation started splitting water molecules into hydrogen and oxygen. Because of Mars' relatively low gravity, it wasn't able to hold onto the very light hydrogen atoms, but the heavier oxygen atoms remained behind.
"Much of this oxygen went into the rocks, leading to the rusty red dust that covers the surface today. While Mars' famous red iron oxides require only a mildly oxidizing environment to form, manganese oxides require a strongly oxidizing environment. These results suggest that past conditions were far more oxidizing (oxygen-rich) than previously thought.
"It's hard to confirm whether this scenario for Martian atmospheric oxygen actually occurred," Lanza added. "But it's important to note that this idea represents a departure in our understanding for how planetary atmospheres might become oxygenated." So far, abundant atmospheric oxygen has been treated as a so-called biosignature, or a sign of existing life.
The next step in this work is for scientists to better understand the signatures of non-biogenic versus biogenic manganese, which is directly produced by microbes. If it's possible to distinguish between manganese oxides produced by life and those produced in a non-biological setting, that knowledge can be directly applied to Martian manganese observations to better understand their origin.
The high-manganese materials were found in mineral-filled cracks in sandstones in the Kimberley region of Gale crater, which the Curiosity rover has been exploring for the last four years. But that's not the only place on Mars that abundant manganese has been found.
The Opportunity rover, which has been exploring Mars since 2004, also recently discovered high-manganese deposits in its landing site thousands of miles from Curiosity, which supports the idea that the conditions needed to form these materials were present well beyond Gale crater.
<a href="http://www.vwkweb.nl/" rel="nofollow" target="_blank">[b]Vereniging voor weerkunde en klimatologie[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>


06-07-2016
Marsjeep Curiosity zet zichzelf op een laag pitje
© reuters.
De Amerikaanse Marsjeep Curiosity heeft zichzelf in een "veilige modus" gezet, zo heeft space.com woensdag gemeld.
De robot schakelde vandaag om een nog onbekende reden over naar een laag pitje. De Marsverkenner communiceert desondanks nog met de vluchtleiding en is stabiel.
In 2013 gebeurde een zelfde scenario drie keer en telkens kwam de succesvolle robot weer helemaal tot leven, aldus space.com.
De 2,4 miljard dollar kostende Curiosity landde in augustus 2012 in de Gale-krater en heeft de kennis over onze buurplaneet grondig bijgespijkerd.
(HLN)
Marsjeep Curiosity zet zichzelf op een laag pitje
© reuters.
De Amerikaanse Marsjeep Curiosity heeft zichzelf in een "veilige modus" gezet, zo heeft space.com woensdag gemeld.
De robot schakelde vandaag om een nog onbekende reden over naar een laag pitje. De Marsverkenner communiceert desondanks nog met de vluchtleiding en is stabiel.
In 2013 gebeurde een zelfde scenario drie keer en telkens kwam de succesvolle robot weer helemaal tot leven, aldus space.com.
De 2,4 miljard dollar kostende Curiosity landde in augustus 2012 in de Gale-krater en heeft de kennis over onze buurplaneet grondig bijgespijkerd.
(HLN)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


15-07-2016
NASA start definitief ontwerp en bouw van Mars 2020 rover
Computerillustratie van NASA's Mars 2020 rover. (NASA/JPL-Caltech)
NASA heeft groen licht gekregen voor het maken van een definitief ontwerp en het starten van de bouw van de Mars 2020 rover. De nieuwe Marswagen moet in de zomer van 2020 gelanceerd worden en zal in februari 2021 op Mars landen.
Het ontwerp lijkt veel op dat van de huidige Marswagen Curiosity. Door aanpassingen van de landingsprocedure zal de nieuwe rover wel in een ruiger terrein kunnen landen dan Curiosity. De gehele afdaling zal vastgelegd worden in beeld en geluid; voor het eerst zullen er ook op het Marsoppervlak zelf geluidsopnamen worden gemaakt.
Belangrijkste doel van de Mars 2020 rover is onderzoek naar fossiele sporen van micro-organismen die lang geleden op Mars geleefd zouden kunnen hebben. In totaal zullen bovendien ca. dertig grond- en bodemmonsters worden verzameld, bestudeerd en verzegeld; de capsules kunnen in de toekomst door een andere ruimtemissie worden opgehaald voor laboratoriumonderzoek op aarde. (GS)
(allesoversterrenkunde)
NASA start definitief ontwerp en bouw van Mars 2020 rover
Computerillustratie van NASA's Mars 2020 rover. (NASA/JPL-Caltech)
NASA heeft groen licht gekregen voor het maken van een definitief ontwerp en het starten van de bouw van de Mars 2020 rover. De nieuwe Marswagen moet in de zomer van 2020 gelanceerd worden en zal in februari 2021 op Mars landen.
Het ontwerp lijkt veel op dat van de huidige Marswagen Curiosity. Door aanpassingen van de landingsprocedure zal de nieuwe rover wel in een ruiger terrein kunnen landen dan Curiosity. De gehele afdaling zal vastgelegd worden in beeld en geluid; voor het eerst zullen er ook op het Marsoppervlak zelf geluidsopnamen worden gemaakt.
Belangrijkste doel van de Mars 2020 rover is onderzoek naar fossiele sporen van micro-organismen die lang geleden op Mars geleefd zouden kunnen hebben. In totaal zullen bovendien ca. dertig grond- en bodemmonsters worden verzameld, bestudeerd en verzegeld; de capsules kunnen in de toekomst door een andere ruimtemissie worden opgehaald voor laboratoriumonderzoek op aarde. (GS)
(allesoversterrenkunde)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


The difference between the three Abrahamic religions:
- Christianity mumbling to the ceiling,
- Judaism mumbling to the wall,
- Islam mumbling to the floor.
- Christianity mumbling to the ceiling,
- Judaism mumbling to the wall,
- Islam mumbling to the floor.


16-08-2016
NASA geeft meer dan 1.000 nieuwe beelden van Mars vrij. En ze zijn wonderlijk
© NASA/JPL/University of Arizona.
Mars in beeld De Mars Reconnaissance Orbiter cirkelt al sinds 2005 rond Mars en trekt geregeld kiekjes van de rode planeet. De lading foto's die NASA deze maand lostte, is extra groot en dat levert wonderlijke beelden op van onze buurplaneet. Een kleine selectie toont alvast het gevarieerde en wonderlijke reliëf van Mars.
Het gebeurt geregeld dat de Mars Reconnaissance Orbiter (MRO) foto's aflevert, maar de huidige lading van 1.035 foto's is uitzonderlijk groot. Alfred McEwen, directeur van het Planetary Image Research Laboratory, vertelde aan Popular Science dat daar twee redenen voor zijn: enerzijds is er elke 26 maanden een optimaal moment voor het verzenden van data tussen de Aarde en Mars, anderzijds beleefde Mars in juli zijn herfstequinox, waardoor de MRO het meest volledige en verlichte beeld van de planeet voor de lens kreeg.
De beelden werden geregistreerd door HiRISE, een camera met hoge resolutie die wetenschappers toelaat om het oppervlak van Mars in detail te bestuderen. Die beelden kunnen bijvoorbeeld helpen om landingsplaatsen uit te zoeken voor toekomstige missies.
U kan alle beelden terugvinden via deze database, maar hier hebt u alvast een kleine voorproefje van de enorme variatie aan wonderlijke foto's.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
(HLN)
NASA geeft meer dan 1.000 nieuwe beelden van Mars vrij. En ze zijn wonderlijk
© NASA/JPL/University of Arizona.
Mars in beeld De Mars Reconnaissance Orbiter cirkelt al sinds 2005 rond Mars en trekt geregeld kiekjes van de rode planeet. De lading foto's die NASA deze maand lostte, is extra groot en dat levert wonderlijke beelden op van onze buurplaneet. Een kleine selectie toont alvast het gevarieerde en wonderlijke reliëf van Mars.
Het gebeurt geregeld dat de Mars Reconnaissance Orbiter (MRO) foto's aflevert, maar de huidige lading van 1.035 foto's is uitzonderlijk groot. Alfred McEwen, directeur van het Planetary Image Research Laboratory, vertelde aan Popular Science dat daar twee redenen voor zijn: enerzijds is er elke 26 maanden een optimaal moment voor het verzenden van data tussen de Aarde en Mars, anderzijds beleefde Mars in juli zijn herfstequinox, waardoor de MRO het meest volledige en verlichte beeld van de planeet voor de lens kreeg.
De beelden werden geregistreerd door HiRISE, een camera met hoge resolutie die wetenschappers toelaat om het oppervlak van Mars in detail te bestuderen. Die beelden kunnen bijvoorbeeld helpen om landingsplaatsen uit te zoeken voor toekomstige missies.
U kan alle beelden terugvinden via deze database, maar hier hebt u alvast een kleine voorproefje van de enorme variatie aan wonderlijke foto's.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
© NASA/JPL/University of Arizona.
(HLN)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


19-08-2016
Curiosity legt Murray Buttes vast in nieuw 360-graden-panorama
De nieuwe panorama-opname van Mars, gemaakt door de Amerikaanse Marswagen Curiosity. (NASA/JPL-Caltech/MSSS)
De Amerikaanse Marswagen Curiosity, die onderzoek doet op de hellingen van Mount Sharp in de grote Marskrater Gale, heeft een nieuw 360-graden-panorama gefotografeerd van zijn omgeving. De opvallendste geologische structuren op de panoramafoto zijn de zogeheten Murray Buttes. Buttes (oorspronkelijk een Frans woord) zijn afgevlakte 'tafelbergen' zoals ze veel voorkomen in het zuidwesten van de Verenigde Staten. Het gaat om sedimentatiegesteenten die in de loop van miljoenen jaren zijn geërodeerd. Op plaatsen waar het gesteente harder is dan normaal vindt de erosie minder snel plaats, en blijven er bergen met een vlakka bovenzijde over.
De zandsteenbuttes die door Curiosity zijn gefotografeerd, zijn genoemd naar Bruce Murray (1931-2013), die lange tijd directeur is geweest van NASA's Jet Propulsion Laboratory in Pasadena. De panoramafoto is samengesteld uit 130 afzonderlijke opnamen die op 5 augustus zijn gemaakt met de Mastcam-camera van Curiosity. (GS)
Hogeresolutieversie van de panorama-foto
(allesoversterrenkunde)
Curiosity legt Murray Buttes vast in nieuw 360-graden-panorama
De nieuwe panorama-opname van Mars, gemaakt door de Amerikaanse Marswagen Curiosity. (NASA/JPL-Caltech/MSSS)
De Amerikaanse Marswagen Curiosity, die onderzoek doet op de hellingen van Mount Sharp in de grote Marskrater Gale, heeft een nieuw 360-graden-panorama gefotografeerd van zijn omgeving. De opvallendste geologische structuren op de panoramafoto zijn de zogeheten Murray Buttes. Buttes (oorspronkelijk een Frans woord) zijn afgevlakte 'tafelbergen' zoals ze veel voorkomen in het zuidwesten van de Verenigde Staten. Het gaat om sedimentatiegesteenten die in de loop van miljoenen jaren zijn geërodeerd. Op plaatsen waar het gesteente harder is dan normaal vindt de erosie minder snel plaats, en blijven er bergen met een vlakka bovenzijde over.
De zandsteenbuttes die door Curiosity zijn gefotografeerd, zijn genoemd naar Bruce Murray (1931-2013), die lange tijd directeur is geweest van NASA's Jet Propulsion Laboratory in Pasadena. De panoramafoto is samengesteld uit 130 afzonderlijke opnamen die op 5 augustus zijn gemaakt met de Mastcam-camera van Curiosity. (GS)
Hogeresolutieversie van de panorama-foto
(allesoversterrenkunde)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


29-08-2016
NASA beëindigt Marssimulatie van één jaar op Hawaï
De koepel waarin de crew woont op een vulkaantop in Hawai. © Twitter: @CyprienVerseux.
Leven op Mars is dichterbij dan je wellicht denkt: zes mensen hebben afgelopen jaar 'daar' doorgebracht. Maar vandaag kon de crew eindelijk hun ruimtevaartpak uittrekken en terugkeren naar de aarde: na bijna een jaar lang in isolatie geleefd te hebben op Hawaï in een simulatie van de 'rode planeet', heeft NASA het experiment beëindigt.
De missie was het langste experiment tot nu toe om de gevolgen te meten van het isolement en de beperkingen die een drie jaar durende vlucht naar de rode planeet zich mee zou brengen. Een crew van vier Amerikanen, een Fransman en een Duitser, woonde samen op zo'n 2.500 meter boven zeeniveau in een kleine koepel (diameter: 11 meter diameter, hoogte: 6 meter) op de rotsachtige grond van de vulkaan Mauna Loa-vulkaan op Hawaï: het dichtste bij een Mars leefomgeving dat we op aarde kunnen komen.
De leefomstandigheden waren zwaar: er was geen frisse lucht, geen vers eten of privacy. Communicatie met de buitenwereld was slechts met een vertraging van 20 minuten (net zoals dat op Mars zou zijn) mogelijk.
Beproevingen
Als de crewleden (een journalist, een bodemspecialist, een astro-bioloog, een natuurkundige, een piloot en een architect) naar buiten wilden gaan, moesten ze zware ruimtepakken aandoen. Om persoonlijke conflicten te vermijden, moesten ze werken en wetenschappelijke experimenten uitvoeren. Om het allemaal nog wat zwaarder te maken, werd de crew onverwachts voor zware 'beproevingen' (variërend van noodsituaties, tot gebroken werkmateriaal) gesteld. Onderzoekers wilden zo de groepscohesie en -prestaties meten. Daarnaast moesten de 'astronauten' wekelijkse vragenlijsten invullen en werden de 'formele vergaderingen in de keuken opgenomen. Crewleden die last hadden van heimwee konden een virtual reality-spelletje spelen dat ontworpen is om astronauten die lange missies uitvoeren te 'troosten'.
Zwemmen
"De onderzoekers kijken vooral uit naar het eten van vers voedsel en ander eten dat ze tijdens het experiment niet kregen. Daarnaast kunnen ze niet wachten om in de oceaan te gaan zwemmen", vertelt Kim Binsted, die de simulatie leidde.
Reis van drie jaar
NASA hoopt om tegen 2030 de eerste mensen naar Mars te kunnen sturen. In voorbereiding daarop, houdt de ruimtevaartorganisatie verschillende 'campagnes' of experimenten waarmee ze een zo goed mogelijk beeld probeert te krijgen van wat de eerste crew nodig heeft om de lange reis te maken. Een enkele reis naar Mars duurt namelijk alleen al een slordig jaar. "Er gaat nooit een 'perfecte' crew zijn", zegt Binsted. "Maar inzichten uit deze experimenten kunnen wel helpen om een zo'n ideaal mogelijke groep mensen samen te stellen. Er zullen altijd dingen misgaan, maar op basis van het materiaal dat we met deze experimenten hebben verzameld hopen we erachter te komen hoe we de intermenselijke problemen het makkelijkste kunnen tackelen onderweg."
Compromissen
Het derde en langste experiment tot nu toe eindigde vandaag: de crewleden werden met de boodschap 'welkom terug op Aarde' uit hun isolement vrijgelaten. Na de missie raadde de Duitse onderzoekster Christiane Heinicke toekomstige Marsreizigers aan om "iets mee te nemen om aan te werken: een van de grootste vijanden is de verveling. Je moet bovendien bereid zijn om je aan te passen aan de anderen en om compromissen te maken. Als je dat niet kunt, dan moet je niet naar Mars gaan."
Droom
De missie op Hawaï was de op één na langste ooit, na een Russische missie die 520 dagen duurde. Er werden eerder al drie korte simulaties uitgevoerd op Hawaï, en er zullen in de toekomst nog twee andere missies plaatsvinden, waarvan er één acht maanden zal duren.
Moest de droom van vele wetenschappers werkelijkheid worden om naar Mars te vliegen, zou de reis naar de Rode Planeet alleen al ongeveer een jaar duren. Experts schatten dat een missie naar Mars één tot drie jaar zou kunnen duren.
(H)
NASA beëindigt Marssimulatie van één jaar op Hawaï
De koepel waarin de crew woont op een vulkaantop in Hawai. © Twitter: @CyprienVerseux.
Leven op Mars is dichterbij dan je wellicht denkt: zes mensen hebben afgelopen jaar 'daar' doorgebracht. Maar vandaag kon de crew eindelijk hun ruimtevaartpak uittrekken en terugkeren naar de aarde: na bijna een jaar lang in isolatie geleefd te hebben op Hawaï in een simulatie van de 'rode planeet', heeft NASA het experiment beëindigt.
De missie was het langste experiment tot nu toe om de gevolgen te meten van het isolement en de beperkingen die een drie jaar durende vlucht naar de rode planeet zich mee zou brengen. Een crew van vier Amerikanen, een Fransman en een Duitser, woonde samen op zo'n 2.500 meter boven zeeniveau in een kleine koepel (diameter: 11 meter diameter, hoogte: 6 meter) op de rotsachtige grond van de vulkaan Mauna Loa-vulkaan op Hawaï: het dichtste bij een Mars leefomgeving dat we op aarde kunnen komen.
De leefomstandigheden waren zwaar: er was geen frisse lucht, geen vers eten of privacy. Communicatie met de buitenwereld was slechts met een vertraging van 20 minuten (net zoals dat op Mars zou zijn) mogelijk.
Beproevingen
Als de crewleden (een journalist, een bodemspecialist, een astro-bioloog, een natuurkundige, een piloot en een architect) naar buiten wilden gaan, moesten ze zware ruimtepakken aandoen. Om persoonlijke conflicten te vermijden, moesten ze werken en wetenschappelijke experimenten uitvoeren. Om het allemaal nog wat zwaarder te maken, werd de crew onverwachts voor zware 'beproevingen' (variërend van noodsituaties, tot gebroken werkmateriaal) gesteld. Onderzoekers wilden zo de groepscohesie en -prestaties meten. Daarnaast moesten de 'astronauten' wekelijkse vragenlijsten invullen en werden de 'formele vergaderingen in de keuken opgenomen. Crewleden die last hadden van heimwee konden een virtual reality-spelletje spelen dat ontworpen is om astronauten die lange missies uitvoeren te 'troosten'.
Zwemmen
"De onderzoekers kijken vooral uit naar het eten van vers voedsel en ander eten dat ze tijdens het experiment niet kregen. Daarnaast kunnen ze niet wachten om in de oceaan te gaan zwemmen", vertelt Kim Binsted, die de simulatie leidde.
Reis van drie jaar
NASA hoopt om tegen 2030 de eerste mensen naar Mars te kunnen sturen. In voorbereiding daarop, houdt de ruimtevaartorganisatie verschillende 'campagnes' of experimenten waarmee ze een zo goed mogelijk beeld probeert te krijgen van wat de eerste crew nodig heeft om de lange reis te maken. Een enkele reis naar Mars duurt namelijk alleen al een slordig jaar. "Er gaat nooit een 'perfecte' crew zijn", zegt Binsted. "Maar inzichten uit deze experimenten kunnen wel helpen om een zo'n ideaal mogelijke groep mensen samen te stellen. Er zullen altijd dingen misgaan, maar op basis van het materiaal dat we met deze experimenten hebben verzameld hopen we erachter te komen hoe we de intermenselijke problemen het makkelijkste kunnen tackelen onderweg."
Compromissen
Het derde en langste experiment tot nu toe eindigde vandaag: de crewleden werden met de boodschap 'welkom terug op Aarde' uit hun isolement vrijgelaten. Na de missie raadde de Duitse onderzoekster Christiane Heinicke toekomstige Marsreizigers aan om "iets mee te nemen om aan te werken: een van de grootste vijanden is de verveling. Je moet bovendien bereid zijn om je aan te passen aan de anderen en om compromissen te maken. Als je dat niet kunt, dan moet je niet naar Mars gaan."
Droom
De missie op Hawaï was de op één na langste ooit, na een Russische missie die 520 dagen duurde. Er werden eerder al drie korte simulaties uitgevoerd op Hawaï, en er zullen in de toekomst nog twee andere missies plaatsvinden, waarvan er één acht maanden zal duren.
Moest de droom van vele wetenschappers werkelijkheid worden om naar Mars te vliegen, zou de reis naar de Rode Planeet alleen al ongeveer een jaar duren. Experts schatten dat een missie naar Mars één tot drie jaar zou kunnen duren.
(H)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


ELON MUSK PLANS TO GET HUMANS TO MARS IN SIX YEARS
SpaceX founder tells meeting of astronautical experts that his only purpose is to ‘make life interplanetary’, revealing plans for reusable ship to Mars
Elon Musk unveils his plans to colonize Mars
SpaceX founder Elon Musk has outlined his highly ambitious vision for manned missions to Mars, which he said could begin as soon as 2022 – three years sooner than his previous estimates.
However, the question of how such extravagantly expensive missions would be funded remains largely in the dark.
Elon Musk has ambitious plans for Mars. Are they as crazy as they sound?
“What I really want to try to achieve here is to make Mars seem possible – like it’s something we can achieve in our lifetimes,” Musk told an audience in his keynote speech at the International Astronautical Congress in Guadalajara, Mexico, on Tuesday.
He said there were “two fundamental paths” facing humanity today. “One is that we stay on Earth forever and then there will be an inevitable extinction event,” he said. “The alternative is to become a spacefaring civilization, and a multi-planetary species.”
A shot of a video about the Interplanetary Transport System, which aims to reach Mars with a human crew for the first time in history
In order to achieve this goal, Musk outlined a multi-stage launch and transport system, including a reusable booster – like the Falcon 9, which SpaceX has already successfully tested – only much larger. The booster, and the “interplanetary module” on top of it, would be nearly as long as two Boeing 747 aircraft. It could initially carry up to 100 passengers, he said.
The first ship to go to Mars, Musk said, would be named Heart of Gold as a tribute to the ship powered by an “infinite improbability drive” from Douglas Adams’ science fiction novel The Hitchhiker’s Guide to the Galaxy.
Similar modules, also launched using reusable boosters, would remain in Earth’s orbit to refuel the interplanetary craft to be able to use multiple trips, including to other parts of the solar system such as Enceladus, a moon of Saturn on which Nasa’s Cassini mission recently found evidence of a polar subsurface water ocean that could harbor life.
Musk also outlined a system by which fuel could be synthesized on Mars from water and carbon dioxide in order to fuel return journeys to Earth.
He estimated the current cost of sending someone to Mars at “around $10bn per person”, though it was not clear if he meant using existing rocket systems or on the initial flight of his proposed system. He said that there would be price improvements over time because of the reusability of the spacecraft, in-orbit refuelling and on-Mars propellant production that would reduce that cost by “orders of magnitude”.
But he made little attempt to solve the thorny problem of the initial cost of constructing the system. Suggesting possible revenue streams, Musk proposed two sources of cash – sending cargo and astronauts to the International Space Station and launching satellites – both already part of SpaceX’s business model.
Elon Musk estimated the current cost of sending someone to Mars at ‘around $10bn per person’
He also listed three other sources of revenue that simply read “kickstarter”, “profit” and – intriguingly – “steal underpants”.
Asked at the talk about funding, however, Musk said: “The reason I am personally accruing assets is to fund this. I really have no other purpose than to make life interplanetary.”
Bill Nye, chief executive officer of the Planetary Society and host of the popular TV show Bill Nye the Science Guy, was in the audience and described the energy of the crowd as “extraordinary”.
“Watching the crowd go absolutely wild today tells me that the best is yet ahead for space exploration,” he told the Guardian, adding that Musk had presented “a very aggressive schedule that seemed feasible to the crowd”.
“No matter what we send to Mars, I very much hope we conduct a thorough, careful search for life before we consider landing people and cargo. I believe the discovery of life or evidence of life would change the way we think about the cosmos and our place within it,” Nye added.
Nasa said in a statement that it welcomed Musk’s plans. “NASA applauds all those who want to take the next giant leap – and advance the journey to Mars. We are very pleased that the global community is working to meet the challenges of a sustainable human presence on Mars. This journey will require the best and the brightest minds from government and industry, and the fact that Mars is a major topic of discussion is very encouraging.”
Nasa says it has made “extraordinary progress” developing a plan for sustainable Mars exploration, building partnerships in both the public and private sectors.
“The fundamental cause of the trouble in the modern world today is that the stupid are cocksure while the intelligent are full of doubt.”— Bertrand Russell


we gaan de volgende planeet verpesten 
DeLuna vindt me dik ;(
Op zondag 22 juni 2014 12:30 schreef 3rdRock het volgende:
pas als jullie gaan trouwen. nu ben je gewoon die Oom Rubber die met onze mama leuke dingen doet :)
Op zondag 22 juni 2014 12:30 schreef 3rdRock het volgende:
pas als jullie gaan trouwen. nu ben je gewoon die Oom Rubber die met onze mama leuke dingen doet :)


03-10-2016
Curiosity zoekt het hogerop
Zelfportret van Curiosity bij de zogeheten Murray Buttes. (NASA/JPL-Caltech/MSSS)
De Amerikaanse Marswagen Curiosity gaat op weg naar hoger gelegen delen op de flanken van Mount Sharp, de centrale berg in de grote Marskrater Gale waar Curiosity vier jaar geleden landde. Op 1 oktober is officieel de afgelopen zomer goedgekeurde missieverlenging van Curiosity begonnen.
De afgelopen tijd bracht Curiosity door bij de zogeheten Murray Buttes - rotsformaties in een gebied dat enkele miljarden jaren geleden lange tijd 'onder water' heeft gestaan, toen de krater Gale een kleine binnenzee bevatte. In september is voor de veertiende maal bodemmateriaal opgeboord voor nader onderzoek aan boord van de Marswagen.
De twee belangrijkste reisdoelen voor de komende tijd zijn 'Hematite Unit' en 'Clay Unit'. Hematite Unit is een rotsformatie op ca. 2,5 kilometer afstand die veel van het ijzerrijke mineraal hematiet bevat. Nog verderop ligt Clay Unit, een gebied met bodemgesteente waarin veel kleiafzettingen voorkomen. Beide gebieden zijn geselecteerd op basis van metingen die verricht zijn door kunstmanen in een baan om Mars.
Curiosity landde in augustus 2012 en heeft sindsdien ca. 180.000 foto's gemaakt, waaronder een aantal spectaculaire 'zelfportretten'. Die zijn opgebouwd uit tientallen afzonderlijke foto's, gemaakt met de camera op het uiteinde van de 'mast' van de Marswagen. Die mast is vervolgens langs digitale weg uit het fotomozaïek verwijderd. (GS)
(allesoversterrenkunde)
Curiosity zoekt het hogerop
Zelfportret van Curiosity bij de zogeheten Murray Buttes. (NASA/JPL-Caltech/MSSS)
De Amerikaanse Marswagen Curiosity gaat op weg naar hoger gelegen delen op de flanken van Mount Sharp, de centrale berg in de grote Marskrater Gale waar Curiosity vier jaar geleden landde. Op 1 oktober is officieel de afgelopen zomer goedgekeurde missieverlenging van Curiosity begonnen.
De afgelopen tijd bracht Curiosity door bij de zogeheten Murray Buttes - rotsformaties in een gebied dat enkele miljarden jaren geleden lange tijd 'onder water' heeft gestaan, toen de krater Gale een kleine binnenzee bevatte. In september is voor de veertiende maal bodemmateriaal opgeboord voor nader onderzoek aan boord van de Marswagen.
De twee belangrijkste reisdoelen voor de komende tijd zijn 'Hematite Unit' en 'Clay Unit'. Hematite Unit is een rotsformatie op ca. 2,5 kilometer afstand die veel van het ijzerrijke mineraal hematiet bevat. Nog verderop ligt Clay Unit, een gebied met bodemgesteente waarin veel kleiafzettingen voorkomen. Beide gebieden zijn geselecteerd op basis van metingen die verricht zijn door kunstmanen in een baan om Mars.
Curiosity landde in augustus 2012 en heeft sindsdien ca. 180.000 foto's gemaakt, waaronder een aantal spectaculaire 'zelfportretten'. Die zijn opgebouwd uit tientallen afzonderlijke foto's, gemaakt met de camera op het uiteinde van de 'mast' van de Marswagen. Die mast is vervolgens langs digitale weg uit het fotomozaïek verwijderd. (GS)
(allesoversterrenkunde)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


Nog 9 dagen...
Zo gaat Schiaparelli van de ExoMars 2016 missie op 19 oktober op Mars landen
8 oktober 2016 door Arie Nouwen.
De Schiaparelli lander van de Europees-Russische ExoMars 2016 missie, op 14 maart dit jaar gelanceerd, zal op woensdag 19 oktober aanstaande om exact 16:48:11 uur Nederlandse tijd landen in het Meridiani Planum gebied bij de evenaar van Mars, de Rode Planeet. Bij de afdaling zal Schiaparelli met een snelheid van 21.000 km per uur de ijle atmosfeer van Mars binnenkomen en dan gebruik makend van z’n hitteschild, parachutes en motoren afremmen en zacht landen. Mmmm, voor de periode rond 29 oktober wordt een grote stofstorm op Mars voorspeld. Niet te hopen dat Schiaparelli daarin verzeild zal raken.
De afdaling en landing lijken op die van de Curiosity, die op 12 augustus 2012 met een Skycrane op Mars landde. Drie dagen eerder, op 16 oktober, zal Schiaparelli zich afscheiden van de Trace Gas Orbiter, die om Mars zal gaan cirkelen en die metingen aan het methaan in de atmosfeer gaat doen. Hieronder een video over de afdaling van Schiaparelli, die ongeveer zes minuten zal gaan duren.
10 october
A stormy arrival for Schiaparelli?
Zo gaat Schiaparelli van de ExoMars 2016 missie op 19 oktober op Mars landen
8 oktober 2016 door Arie Nouwen.
De Schiaparelli lander van de Europees-Russische ExoMars 2016 missie, op 14 maart dit jaar gelanceerd, zal op woensdag 19 oktober aanstaande om exact 16:48:11 uur Nederlandse tijd landen in het Meridiani Planum gebied bij de evenaar van Mars, de Rode Planeet. Bij de afdaling zal Schiaparelli met een snelheid van 21.000 km per uur de ijle atmosfeer van Mars binnenkomen en dan gebruik makend van z’n hitteschild, parachutes en motoren afremmen en zacht landen. Mmmm, voor de periode rond 29 oktober wordt een grote stofstorm op Mars voorspeld. Niet te hopen dat Schiaparelli daarin verzeild zal raken.
De afdaling en landing lijken op die van de Curiosity, die op 12 augustus 2012 met een Skycrane op Mars landde. Drie dagen eerder, op 16 oktober, zal Schiaparelli zich afscheiden van de Trace Gas Orbiter, die om Mars zal gaan cirkelen en die metingen aan het methaan in de atmosfeer gaat doen. Hieronder een video over de afdaling van Schiaparelli, die ongeveer zes minuten zal gaan duren.
10 october
A stormy arrival for Schiaparelli?
<a href="http://www.vwkweb.nl/" rel="nofollow" target="_blank">[b]Vereniging voor weerkunde en klimatologie[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>


countdown continues 
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


11-10-2016
Obama wil mensen op Mars laten wonen: "Amerika zal die reuzensprong maken"
Amerika's "verhaal in de ruimte" moet een nieuw hoofdstuk krijgen: een bemande missie naar Mars. Dat doel heeft de Amerikaanse president Barack Obama vandaag aangekondigd. In de jaren dertig van deze eeuw moeten wat hem betreft voor het eerst mensen naar Mars reizen en veilig terugkeren. "Amerika zal deze reuzensprong maken", klinkt het.
Op de website van CNN betoogde Obama dat ruimtevaart uiteindelijk het leven op aarde beter maakt. "Als we ons leiderschap in de ruimte deze eeuw nog sterker maken dan in de volgende, zullen we niet alleen profijt hebben van de daarmee gepaard gaande vooruitgang op het gebied van energie, gezondheidszorg, landbouw en kunstmatige intelligentie. We zullen ook een betere kennis krijgen van onze omgeving en onszelf".
Kinderlijke verwondering
"Ik ervaar nog altijd dezelfde verwondering over ons ruimteprogramma als ik deed toen ik een kind was. Het staat voor een wezenlijk element van onze aard: nieuwsgierigheid en verkenning, vernieuwing en vindingrijkheid, grensverleggend en baanbrekend. De race in de ruimte die we hebben gewonnen heeft niet enkel enorm bijgedragen aan belangrijke technologische en medische ontwikkelingen, maar heeft ook een nieuwe generatie wetenschappers en technici geïnspireerd", aldus Obama in de mededeling.
De missies naar Mars moeten voortkomen uit samenwerking tussen de overheid en de private sector.
Obama schrijft dat hij opgetogen is', omdat de Amerikaanse overheid "samenwerkt met commerciële partners om nieuwe habitats te bouwen, die astronauten op langdurige missies in de ruimte in leven kunnen houden.''
Elon Musk
In het verleden zijn al vaak plannen gemaakt voor bemande missies naar de rode planeet, maar geen daarvan werd tot nog toe uitgevoerd. Momenteel zijn er diverse initiatieven gaande om de fantasie werkelijkheid te laten worden. Ondernemer Elon Musk ontvouwde onlangs de plannen van zijn bedrijf SpaceX om al in 2024 de eerste mensen naar Mars te brengen.
(HLN)
Obama wil mensen op Mars laten wonen: "Amerika zal die reuzensprong maken"
Amerika's "verhaal in de ruimte" moet een nieuw hoofdstuk krijgen: een bemande missie naar Mars. Dat doel heeft de Amerikaanse president Barack Obama vandaag aangekondigd. In de jaren dertig van deze eeuw moeten wat hem betreft voor het eerst mensen naar Mars reizen en veilig terugkeren. "Amerika zal deze reuzensprong maken", klinkt het.
Op de website van CNN betoogde Obama dat ruimtevaart uiteindelijk het leven op aarde beter maakt. "Als we ons leiderschap in de ruimte deze eeuw nog sterker maken dan in de volgende, zullen we niet alleen profijt hebben van de daarmee gepaard gaande vooruitgang op het gebied van energie, gezondheidszorg, landbouw en kunstmatige intelligentie. We zullen ook een betere kennis krijgen van onze omgeving en onszelf".
Kinderlijke verwondering
"Ik ervaar nog altijd dezelfde verwondering over ons ruimteprogramma als ik deed toen ik een kind was. Het staat voor een wezenlijk element van onze aard: nieuwsgierigheid en verkenning, vernieuwing en vindingrijkheid, grensverleggend en baanbrekend. De race in de ruimte die we hebben gewonnen heeft niet enkel enorm bijgedragen aan belangrijke technologische en medische ontwikkelingen, maar heeft ook een nieuwe generatie wetenschappers en technici geïnspireerd", aldus Obama in de mededeling.
De missies naar Mars moeten voortkomen uit samenwerking tussen de overheid en de private sector.
Obama schrijft dat hij opgetogen is', omdat de Amerikaanse overheid "samenwerkt met commerciële partners om nieuwe habitats te bouwen, die astronauten op langdurige missies in de ruimte in leven kunnen houden.''
Elon Musk
In het verleden zijn al vaak plannen gemaakt voor bemande missies naar de rode planeet, maar geen daarvan werd tot nog toe uitgevoerd. Momenteel zijn er diverse initiatieven gaande om de fantasie werkelijkheid te laten worden. Ondernemer Elon Musk ontvouwde onlangs de plannen van zijn bedrijf SpaceX om al in 2024 de eerste mensen naar Mars te brengen.
(HLN)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


11-10-2016
Kosmische straling zal astronauten naar Mars dement en paranoïde maken
Mars-simulatie op de Oostenrijkse Kaunertal gletsjer. © epa.
Amerikaans president Obama wil mensen op Mars laten wonen, zo maakte hij vandaag bekend. "Die reuzensprong zal Amerika maken", liet hij weten. Vrijwilligers genoeg, alleen moeten die weten dat ze door de kosmische straling risico lopen dement en/of paranoïde te worden. Daarvoor waarschuwt een nieuwe studie.
Astronauten op weg naar Mars kunnen dementie ontwikkelen, naast een onbeheersbaar angstgevoel dat de onderzoekers 'space brain' noemen. Wetenschappers onderzochten de effecten van de kosmische straling waarmee astronauten zullen gebombardeerd worden. Die blijkt een probleem te vormen voor wie een buitenaardse kolonie zou willen stichten op de Rode Planeet.
NASA onderzoekt momenteel hoe het mensen naar Mars kan sturen, en de Nederlandse groep Mars One plant de eerste bemande Marsvlucht tegen 2027. De Amerikaanse ondernemer Elon Musk wil tegen 2022 mensen naar Mars sturen.
Angstbestrijdingsproces
Professor Charles Limoli, expert in stralingsoncologie aan de universiteit van Californië, stelde samen met zijn collega's vast dat deeltjes in die kosmische straling op lange termijn bij muizen voor verminderde hersenfuncties en dementie leiden. De straling bleek ook in te werken op het zogenaamde 'angstbestrijdingsproces', dat de mens helpt angsaanjagende incidenten te overwinnen zodat hij bijvoorbeeld opnieuw kan gaan zwemmen na bijna verdronken te zijn. Dat stelt Limoli in het vakblad Scientific Reports.
"Dat is geen goed nieuws voor astronauten die een reis van twee tot drie jaar naar Mars aanvatten", besluit hij.
Levenslang
"De ruimte stelt de astronauten voor unieke gevaren. Blootstelling aan die stralingspartikels kan leiden tot een hele reeks complicaties in het centrale zenuwstelsel, tijdens de ruimtereis en lang daarna. Het gaat dan om de afname van prestaties, geheugenverlies, angsten, depressies en verzwakte besluitvorming. Die kunnen levenslang aanhouden".
Paniek
De eerste Marsverkenners riskeren ook nog tijdens de vlucht aan paranoïa ten prooi te vallen door het effect op het hersenproces dat afrekent met stresserende gebeurtenissen. "Die verminderde angstbestrijding maakt je vatbaar voor paniek, wat voor problemen kan zorgen tijdens zo'n lange ruimtereis", stelt Limoli.
Astronauten hebben dan wel al langer dan een jaar in het International Space Station (ISS) verbleven, daar werden ze niet aan dezelfde mate van kosmische straling blootgesteld omdat ze in de beschermende magnetosfeer van de Aarde zitten.
"Niet aan te ontsnappen"
Delen van het ruimtetuig zouden extra schilden tegen kosmische stralen kunnen krijgen, maar dat volstaat vooralsnog niet om de astronauten te beschermen. "Er valt niet aan de kosmische stralen te ontsnappen", meent Limoli. Hij en zijn team werken aan een medicijn dat bescherming zou bieden tegen de zwaarste effecten van de straling.
(HLN)
Kosmische straling zal astronauten naar Mars dement en paranoïde maken
Mars-simulatie op de Oostenrijkse Kaunertal gletsjer. © epa.
Amerikaans president Obama wil mensen op Mars laten wonen, zo maakte hij vandaag bekend. "Die reuzensprong zal Amerika maken", liet hij weten. Vrijwilligers genoeg, alleen moeten die weten dat ze door de kosmische straling risico lopen dement en/of paranoïde te worden. Daarvoor waarschuwt een nieuwe studie.
Astronauten op weg naar Mars kunnen dementie ontwikkelen, naast een onbeheersbaar angstgevoel dat de onderzoekers 'space brain' noemen. Wetenschappers onderzochten de effecten van de kosmische straling waarmee astronauten zullen gebombardeerd worden. Die blijkt een probleem te vormen voor wie een buitenaardse kolonie zou willen stichten op de Rode Planeet.
NASA onderzoekt momenteel hoe het mensen naar Mars kan sturen, en de Nederlandse groep Mars One plant de eerste bemande Marsvlucht tegen 2027. De Amerikaanse ondernemer Elon Musk wil tegen 2022 mensen naar Mars sturen.
Angstbestrijdingsproces
Professor Charles Limoli, expert in stralingsoncologie aan de universiteit van Californië, stelde samen met zijn collega's vast dat deeltjes in die kosmische straling op lange termijn bij muizen voor verminderde hersenfuncties en dementie leiden. De straling bleek ook in te werken op het zogenaamde 'angstbestrijdingsproces', dat de mens helpt angsaanjagende incidenten te overwinnen zodat hij bijvoorbeeld opnieuw kan gaan zwemmen na bijna verdronken te zijn. Dat stelt Limoli in het vakblad Scientific Reports.
"Dat is geen goed nieuws voor astronauten die een reis van twee tot drie jaar naar Mars aanvatten", besluit hij.
Levenslang
"De ruimte stelt de astronauten voor unieke gevaren. Blootstelling aan die stralingspartikels kan leiden tot een hele reeks complicaties in het centrale zenuwstelsel, tijdens de ruimtereis en lang daarna. Het gaat dan om de afname van prestaties, geheugenverlies, angsten, depressies en verzwakte besluitvorming. Die kunnen levenslang aanhouden".
Paniek
De eerste Marsverkenners riskeren ook nog tijdens de vlucht aan paranoïa ten prooi te vallen door het effect op het hersenproces dat afrekent met stresserende gebeurtenissen. "Die verminderde angstbestrijding maakt je vatbaar voor paniek, wat voor problemen kan zorgen tijdens zo'n lange ruimtereis", stelt Limoli.
Astronauten hebben dan wel al langer dan een jaar in het International Space Station (ISS) verbleven, daar werden ze niet aan dezelfde mate van kosmische straling blootgesteld omdat ze in de beschermende magnetosfeer van de Aarde zitten.
"Niet aan te ontsnappen"
Delen van het ruimtetuig zouden extra schilden tegen kosmische stralen kunnen krijgen, maar dat volstaat vooralsnog niet om de astronauten te beschermen. "Er valt niet aan de kosmische stralen te ontsnappen", meent Limoli. Hij en zijn team werken aan een medicijn dat bescherming zou bieden tegen de zwaarste effecten van de straling.
(HLN)
Death Makes Angels of us all
And gives us wings where we had shoulders
Smooth as raven' s claws...
And gives us wings where we had shoulders
Smooth as raven' s claws...


Ik snap niet dat de maan een goede optie is.
Helium 3, dichtbij de aarde, maanzand is makkelijk te gebruiken voor het maken van beton..
Mars heeft dit allemaal niet. Vooral de afstand is ruk.
Helium 3, dichtbij de aarde, maanzand is makkelijk te gebruiken voor het maken van beton..
Mars heeft dit allemaal niet. Vooral de afstand is ruk.


Op Mars kan je uiteindelijk zelfvoorzienend worden maar de maan lijkt mij ook makkelijker inderdaad om mee te beginnen.quote:Op woensdag 12 oktober 2016 10:36 schreef k3vil het volgende:
Ik snap niet dat de maan een goede optie is.
Helium 3, dichtbij de aarde, maanzand is makkelijk te gebruiken voor het maken van beton..
Mars heeft dit allemaal niet. Vooral de afstand is ruk.


Geen atmosfeer....quote:Op woensdag 12 oktober 2016 10:36 schreef k3vil het volgende:
Ik snap niet dat de maan een goede optie is.
Helium 3, dichtbij de aarde, maanzand is makkelijk te gebruiken voor het maken van beton..
Mars heeft dit allemaal niet. Vooral de afstand is ruk.
mars willen ze op den duur gaan terraformen
En de afstand (reistijd) is idd groot.
Maar in de toekomst komen raketmotoren
die de reistijd verkorten
<a href="http://www.vwkweb.nl/" rel="nofollow" target="_blank">[b]Vereniging voor weerkunde en klimatologie[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>
<a href="http://www.estofex.org/" rel="nofollow" target="_blank">[b]ESTOFEX[/b]</a>


 Possible liquid water sites on Mars? I might get to investigate two on the surface from afar
Possible liquid water sites on Mars? I might get to investigate two on the surface from afar