Hier een goeie verzameling van onderzoek dat gedaan is naar het gebruik van Hydroxychloroquine:
https://docs.google.com/d(...)XrJl6N1aDjY/preview#
Sequential CQ / HCQ Research Papers and Reports
January to April 20, 2020
Executive Summary Interpretation of the Data In This Report
The HCQ-AZ combination, when started immediately after diagnosis, appears to be a safe and efficient treatment for COVID-19, with a mortality rate of 0.5%, in elderly patients. It avoids worsening and clears virus persistence and contagious infectivity in most cases.
22 August 2005
CDC Special Pathogens Branch
MJ VIncet, E.Bergon, S. Benjannet, BR Erickson, Pierre Rollin, T.G. Ksiazek, NG Seidah,
ST Nichole. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal. (2005) 2: 69
Chloroquine has strong antiviral effects on SARS CoV infection of primate cells in tissue culture. These inhibitory effects are observed when cells are treated with the drug either before or after exposure to the virus, suggesting both prophylactic preventative and treatment use. The paper describes three mechanisms by which the drug might work and suggest it may have both a prophylactic and therapeutic role in Coronavirus infections.
28 January 2020
M. Wang, R. Cao, L. Zhang, X. Yang, J. Liu, M. Xu, Z. Shi, Z. Hu, W. Zhong, G. Xiao
LETTER TO THE EDITOR Cell Research Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research (2020) 0:1–3; https://doi.org/10.1038/s41422-020-0282-0
Tested Remdesivir and Chloroquine in addition to five other drugs were tested in tissue culture against a clinical sample of virus from a COVID-19 patient, Remdesivir and Chloroquine are highly effective in the control of 2019-nCoV infection in vitro. Since these compounds have been used in human patients with a safety track record and shown to be effective against various ailments, we suggest that they should be assessed in human patients suffering from the novel coronavirus disease.
February 13, 2020
Physicians work out treatment guidelines for coronavirus, Korea Biomedical Review http://www.koreabiomed.com/news/articleView.html?idxno=7428
The Korean COVID-19 Central Clinical Task Force, held the sixth video conference and agreed on treatment principles for patients with COVID-19.
Young with mild symptoms without underlying conditions, doctors can observe them without antiviral treatment.
If 10 days have passed since the onset of the illness and the symptoms are mild, physicians do not have to start an antiviral medication.
If patients are old or have underlying conditions with serious symptoms, physicians should consider an antiviral treatment as soon as possible. lopinavir 400mg/ritonavir 100mg (Kaletra two tablets, twice a day) or chloroquine 500mg orally per day. Alternate is hydroxychloroquine 400mg orally per day.
February 18, 2020.
Jianjun Gao, Zhenxue Tian, Xu Yang Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends Advance Publication, DOI: 10.5582/bst.2020.0104
Thus far, results from more than 100 patients have demonstrated that chloroquine phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus negative conversion, and shortening the disease course.
Severe adverse reactions to chloroquine phosphate were not noted in the aforementioned patients. Given these findings, a conference was held on February 15, 2020; participants including experts from government and regulatory authorities and organizers of clinical trials reached an agreement that chloroquine phosphate has potent activity against COVID-19.
27 February 2020
Philippe Colson , Jean-Marc Rolain , Jean-Christophe Lagier , Philippe Brouqui , Didier Raoult , Chloroquine and hydroxychloroquine as available weapons to fight COVID-19, International Journal of Antimicrobial Agents Feb (2020), doi: https://doi.org/10.1016/j.ijantimicag.
2020.105932
following the very recent publication of results showing the in vitro activity of chloroquine against SARS-CoV-2, data have been reported on the efficacy of this drug in patients with SARS-CoV-2-related pneumonia (named COVID-19) at different levels of severity.
Following the in vitro results, 20 clinical studies were launched in several Chinese hospitals.
The first results obtained from more than 100 patients showed the superiority of chloroquine compared with treatment of the control group in terms of reduction of exacerbation of pneumonia, duration of symptoms and delay of viral clearance, all in the absence of severe side effects. This has led in China to include chloroquine in the recommendations regarding the prevention and treatment of COVID-19 pneumonia.
Chinese teams showed that Chloroquine could reduce the length of hospital stay and improve the evolution of COVID-19 pneumonia, leading to recommend the administration of 500 mg of chloroquine twice a day in patients with mild, moderate and severe forms of COVID-19 pneumonia.
4 March 2020
Philippe Colson,a,b Jean-Marc Rolain,a,b Jean-Christophe Lagier,a,b Philippe Brouqui,a,b and Didier Raoult, Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 Mar 4 : 105932. doi: 10.1016/j.ijantimicag.2020.105932 [Epub ahead of print] PMCID: PMC7135139 IPMID: 32145363
A review of the safety and efficiency of CQ and HCQ reviewing more than 20 clinical studies in several Chinese hospitals.
Although only available in letter form, this data caused China to recommend Chloroquine in the National Guidelines for the Treatment of COVID-19.
9 March 2020
X.Yao, F/ Ye2, M. Zhang, C.Cui, R. Lu, H. Li, W. Tan, D. Liu. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). 2020.. Clin Infect Dis. 2020 Mar 9. pii: ciaa237. doi: 10.1093/cid/ciaa237.
Hydroxychloroquine was found to be more potent than chloroquine at inhibiting SARS-CoV-2 in vitro. Hydroxychloroquine sulfate 400 mg given twice daily for 1 day, followed by 200 mg twice daily for 4 more days is recommended to treat SARS-CoV-2 infection.
9 March 2020
Expert Chinese consensus on Chloroquine Phosphate for New Coronavirus Pneumonia. Diagnosis and Treatment Plan. Chinese Journal of Tuberculosis and Respiratory Diseases. 2020, 43:
A Multicenter Collaboration Group was formed to guide and standardize the use of Chloroquine in Coronavirus pneumonia, standardizing Chloroquine treatment at 500mg 2x day for 10 days. Use of azithromycin was contraindicated.
Etc. etc.: https://docs.google.com/d(...)XrJl6N1aDjY/preview#
https://docs.google.com/d(...)XrJl6N1aDjY/preview#
Sequential CQ / HCQ Research Papers and Reports
January to April 20, 2020
Executive Summary Interpretation of the Data In This Report
The HCQ-AZ combination, when started immediately after diagnosis, appears to be a safe and efficient treatment for COVID-19, with a mortality rate of 0.5%, in elderly patients. It avoids worsening and clears virus persistence and contagious infectivity in most cases.
22 August 2005
CDC Special Pathogens Branch
MJ VIncet, E.Bergon, S. Benjannet, BR Erickson, Pierre Rollin, T.G. Ksiazek, NG Seidah,
ST Nichole. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal. (2005) 2: 69
Chloroquine has strong antiviral effects on SARS CoV infection of primate cells in tissue culture. These inhibitory effects are observed when cells are treated with the drug either before or after exposure to the virus, suggesting both prophylactic preventative and treatment use. The paper describes three mechanisms by which the drug might work and suggest it may have both a prophylactic and therapeutic role in Coronavirus infections.
28 January 2020
M. Wang, R. Cao, L. Zhang, X. Yang, J. Liu, M. Xu, Z. Shi, Z. Hu, W. Zhong, G. Xiao
LETTER TO THE EDITOR Cell Research Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research (2020) 0:1–3; https://doi.org/10.1038/s41422-020-0282-0
Tested Remdesivir and Chloroquine in addition to five other drugs were tested in tissue culture against a clinical sample of virus from a COVID-19 patient, Remdesivir and Chloroquine are highly effective in the control of 2019-nCoV infection in vitro. Since these compounds have been used in human patients with a safety track record and shown to be effective against various ailments, we suggest that they should be assessed in human patients suffering from the novel coronavirus disease.
February 13, 2020
Physicians work out treatment guidelines for coronavirus, Korea Biomedical Review http://www.koreabiomed.com/news/articleView.html?idxno=7428
The Korean COVID-19 Central Clinical Task Force, held the sixth video conference and agreed on treatment principles for patients with COVID-19.
Young with mild symptoms without underlying conditions, doctors can observe them without antiviral treatment.
If 10 days have passed since the onset of the illness and the symptoms are mild, physicians do not have to start an antiviral medication.
If patients are old or have underlying conditions with serious symptoms, physicians should consider an antiviral treatment as soon as possible. lopinavir 400mg/ritonavir 100mg (Kaletra two tablets, twice a day) or chloroquine 500mg orally per day. Alternate is hydroxychloroquine 400mg orally per day.
February 18, 2020.
Jianjun Gao, Zhenxue Tian, Xu Yang Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioScience Trends Advance Publication, DOI: 10.5582/bst.2020.0104
Thus far, results from more than 100 patients have demonstrated that chloroquine phosphate is superior to the control treatment in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus negative conversion, and shortening the disease course.
Severe adverse reactions to chloroquine phosphate were not noted in the aforementioned patients. Given these findings, a conference was held on February 15, 2020; participants including experts from government and regulatory authorities and organizers of clinical trials reached an agreement that chloroquine phosphate has potent activity against COVID-19.
27 February 2020
Philippe Colson , Jean-Marc Rolain , Jean-Christophe Lagier , Philippe Brouqui , Didier Raoult , Chloroquine and hydroxychloroquine as available weapons to fight COVID-19, International Journal of Antimicrobial Agents Feb (2020), doi: https://doi.org/10.1016/j.ijantimicag.
2020.105932
following the very recent publication of results showing the in vitro activity of chloroquine against SARS-CoV-2, data have been reported on the efficacy of this drug in patients with SARS-CoV-2-related pneumonia (named COVID-19) at different levels of severity.
Following the in vitro results, 20 clinical studies were launched in several Chinese hospitals.
The first results obtained from more than 100 patients showed the superiority of chloroquine compared with treatment of the control group in terms of reduction of exacerbation of pneumonia, duration of symptoms and delay of viral clearance, all in the absence of severe side effects. This has led in China to include chloroquine in the recommendations regarding the prevention and treatment of COVID-19 pneumonia.
Chinese teams showed that Chloroquine could reduce the length of hospital stay and improve the evolution of COVID-19 pneumonia, leading to recommend the administration of 500 mg of chloroquine twice a day in patients with mild, moderate and severe forms of COVID-19 pneumonia.
4 March 2020
Philippe Colson,a,b Jean-Marc Rolain,a,b Jean-Christophe Lagier,a,b Philippe Brouqui,a,b and Didier Raoult, Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 Mar 4 : 105932. doi: 10.1016/j.ijantimicag.2020.105932 [Epub ahead of print] PMCID: PMC7135139 IPMID: 32145363
A review of the safety and efficiency of CQ and HCQ reviewing more than 20 clinical studies in several Chinese hospitals.
Although only available in letter form, this data caused China to recommend Chloroquine in the National Guidelines for the Treatment of COVID-19.
9 March 2020
X.Yao, F/ Ye2, M. Zhang, C.Cui, R. Lu, H. Li, W. Tan, D. Liu. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). 2020.. Clin Infect Dis. 2020 Mar 9. pii: ciaa237. doi: 10.1093/cid/ciaa237.
Hydroxychloroquine was found to be more potent than chloroquine at inhibiting SARS-CoV-2 in vitro. Hydroxychloroquine sulfate 400 mg given twice daily for 1 day, followed by 200 mg twice daily for 4 more days is recommended to treat SARS-CoV-2 infection.
9 March 2020
Expert Chinese consensus on Chloroquine Phosphate for New Coronavirus Pneumonia. Diagnosis and Treatment Plan. Chinese Journal of Tuberculosis and Respiratory Diseases. 2020, 43:
A Multicenter Collaboration Group was formed to guide and standardize the use of Chloroquine in Coronavirus pneumonia, standardizing Chloroquine treatment at 500mg 2x day for 10 days. Use of azithromycin was contraindicated.
Etc. etc.: https://docs.google.com/d(...)XrJl6N1aDjY/preview#


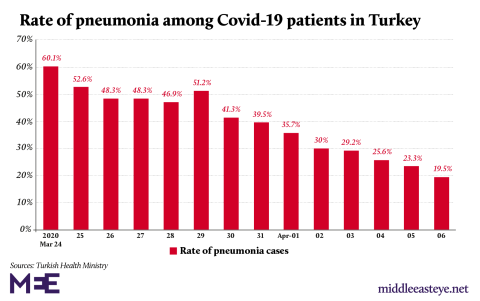
Als je wil weten of Hydroxychrloroquine (HC) werkt moet je naar Turkije kijken. In Turkije krijgen alle CoronapatiŰnten hydroxychloroquine.
In Turkije wordt er in een vroeg stadium HC toegediend. Turkije geeft aan dat sinds ze hiermee begonnen zijn het percentage CoronapatiŰnten dat opgenomen wordt is gezakt van 60% naar 20%.
Ook is het aantal patiŰnten dat op de IC is beland stabiel gebleven terwijl het aantal patiŰnten sterk is gestegen. Dit wordt ook door een arts in de video bevestigd. Dit is een reportage van BBC.
Hier is een artikel van CBS dat wel kritisch is.
https://www.cbsnews.com/n(...)reatment-turkey/#app
Een Turks artikel
https://www.middleeasteye(...)a-treatment-progress
Het gaat trouwens om percentage patienten met longontsteking dat is gedaald.
[ Bericht 0% gewijzigd door polderturk op 01-05-2020 17:16:18 ]
In Turkije wordt er in een vroeg stadium HC toegediend. Turkije geeft aan dat sinds ze hiermee begonnen zijn het percentage CoronapatiŰnten dat opgenomen wordt is gezakt van 60% naar 20%.
Ook is het aantal patiŰnten dat op de IC is beland stabiel gebleven terwijl het aantal patiŰnten sterk is gestegen. Dit wordt ook door een arts in de video bevestigd. Dit is een reportage van BBC.
Hier is een artikel van CBS dat wel kritisch is.
https://www.cbsnews.com/n(...)reatment-turkey/#app
Een Turks artikel
https://www.middleeasteye(...)a-treatment-progress
Het gaat trouwens om percentage patienten met longontsteking dat is gedaald.
[ Bericht 0% gewijzigd door polderturk op 01-05-2020 17:16:18 ]


Interessantquote:Op donderdag 30 april 2020 20:59 schreef polderturk het volgende:
Als je wil weten of Hydroxychrloroquine (HC) werkt moet je naar Turkije kijken. In Turkije krijgen alle CoronapatiŰnten hydroxychloroquine.
In Turkije wordt er in een vroeg stadium HC toegediend. Turkije geeft aan dat sinds ze hiermee begonnen zijn het percentage CoronapatiŰnten dat opgenomen wordt is gezakt van 60% naar 20%.
Ook is het aantal patiŰnten dat op de IC is beland stabiel gebleven terwijl het aantal patiŰnten sterk is gestegen. Dit wordt ook door een arts in de video bevestigd. Dit is een reportage van BBC.
Hier is een artikel van CBS die wel kritisch is.
https://www.cbsnews.com/n(...)reatment-turkey/#app
Een Turks artikel
https://www.middleeasteye(...)a-treatment-progress
Het gaat trouwens om percentage patienten met longontsteking dat is gedaald.
[ afbeelding ]


Ieder huishouden een pakje hydro door de bus voor het geval je Corona verschijnselen krijgt en we kunnen uit lockdown!


In vitro lijkt het inderdaad te werken.
En nu dan in vivo:
En nu dan in vivo:
J. Magagnoli et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv.org. April 21, 2020. doi: 10.1101/2020.04.16.20065920.quote:CONCLUSIONS: In this study, we found no evidence that use of hydroxychloroquine, either with or without azithromycin, reduced the risk of mechanical ventilation in patients hospitalized with Covid-19. An association of increased overall mortality was identified in patients treated with hydroxychloroquine alone. These findings highlight the importance of awaiting the results of ongoing prospective, randomized, controlled studies before widespread adoption of these drugs.
A. Shamshirian et al. Hydroxychloroquine versus COVID-19: A rapid systematic review and meta-analysis. medRxiv.org. April 20, 2020. doi: 10.1101/2020.04.14.20065276.quote:The results of meta-analysis of clinical trials showed that there were no significant differences between patients who received the standard treatment with HCQ regimen and the patients that received the standard treatment without HCQ (RR: 1.44, 95% CI, 0.80-2.59). CT-Scan findings improved in 59% (95% CI 0.15-0.92) and nasopharyngeal culture following RT-PCR resulted negative in 76% (95% CI 0.56-0.89) of patients received hydroxychloroquine. Meta-analysis of observational studies showed 75% (95% CI, 0.54-0.88) of patients were discharged from the hospital, 34% (95% CI, 0.07-0.14) admitted to intensive care unit and 1.5% (95% CI, 0.03-0.83) have expired. Conclusion: This study indicated no clinical benefits regarding HCQ for treatment of COVID-19 patients. However, further large clinical trials should be taken into account in order to achieve more reliable findings.
They told me all of my cages were mental, so I got wasted like all my potential.


Volgens het Turkse RIVM werkt het beter als je het in een vroeg stadium toedient dan wanneer iemand aan een ventilator hangt.quote:


Ik lees hier alleen niets over een kuur van HCQ icm zink. Er zijn internationaal een aantal artsen die beweren succes te hebben met de zink toevoeging. Ben benieuwd of daar ook rekening mee wordt gehouden in de trials.quote:Op vrijdag 1 mei 2020 17:11 schreef speknek het volgende:
In vitro lijkt het inderdaad te werken.
En nu dan in vivo:
[..]
J. Magagnoli et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19. medRxiv.org. April 21, 2020. doi: 10.1101/2020.04.16.20065920.
[..]
A. Shamshirian et al. Hydroxychloroquine versus COVID-19: A rapid systematic review and meta-analysis. medRxiv.org. April 20, 2020. doi: 10.1101/2020.04.14.20065276.


Ja het punt is natuurlijk dat sowieso niet zo heel veel mensen aan de ventilator moeten, dus je weet niet meteen of de HCQ hier effect heeft.quote:Op vrijdag 1 mei 2020 17:15 schreef polderturk het volgende:
[..]
Volgens het Turkse RIVM werkt het beter als je het in een vroeg stadium toedient dan wanneer iemand aan een ventilator hangt.
They told me all of my cages were mental, so I got wasted like all my potential.
|
|